What is Benzoyl Peroxide?
Benzoyl peroxide is an organic compound which has the chemical formula C14H10O4.
It can be prepared from the reaction between benzoyl chloride and hydrogen peroxide. The compound consists of two benzoyl groups that are linked by a peroxide group. Benzoyl peroxide is known to have antibacterial properties and is widely used in the treatment of acne.
Benzoyl Peroxide Structure
The structure of a benzoyl peroxide molecule is illustrated below.
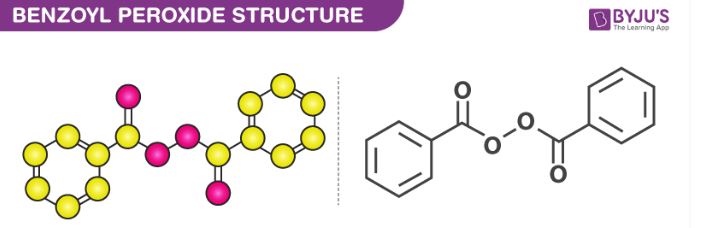
From the illustration provided above, it can be observed that the peroxide group consisting of a single bond between two oxygen atoms links the two benzoyl groups. Therefore, the chemical formula of benzoyl peroxide can also be written as (C6H5CO)2O2.
Due to the weak nature of the oxygen-oxygen bond in the peroxide group that connects the two C6H5CO groups, benzoyl peroxide tends to undergo homolytic fission and form two separate free radical species. The chemical equation for this reaction is provided below.
(C6H5CO)2O2 → 2C6H5CO2•
These free radical species have a very reactive nature due to the presence of an unpaired electron. The formation of these radicals via the homolysis of benzoyl peroxide is encouraged by an increase in temperature.
Table of Contents
- Properties of Benzoyl Peroxide
- Physical Properties
- Chemical Properties
- Uses of Benzoyl Peroxide
- Frequently Asked Questions – FAQs
Properties of Benzoyl Peroxide
The physical and chemical properties of benzoyl peroxide are discussed in this subsection.
Chemical Data of Benzoyl Peroxide
| Chemical Formula | C14H10O4 |
| IUPAC Name | Benzoic Peroxyanhydride |
| Common Name | Benzoyl Peroxide |
| Molar Mass/Molecular Weight | 242.23 grams per mole |
| Melting Point | 103oC |
Physical Properties of Benzoyl Peroxide
- Benzoyl peroxide exists as a colourless solid which has a crystalline structure.
- It has a faint odour which resembles the smell of benzaldehyde.
- C14H10O4 is insoluble in water, somewhat soluble in alcohols, and quite soluble in ethers and chloroform.
- Solid benzoyl peroxide is very volatile and dangerous, especially when dry. It is highly reactive and tends to spontaneously explode.
- At temperatures approaching 103oC, this compound decomposes.
Chemical Properties of Benzoyl Peroxide
- C14H10O4 is an explosive, flammable chemical.
- It is also regarded as an irritant since it can even cause swelling of the skin.
- Benzoyl peroxide can be prepared via the treatment of benzoyl chloride with barium peroxide.
- This compound readily undergoes homolytic fission to yield free radical products that are highly reactive.
- Upon contact with human skin, it breaks down to yield benzoic acid and oxygen.
Uses of Benzoyl Peroxide
Benzoyl peroxide is a very important organic peroxide due to its wide range of applications. It is also produced on a large scale industrially. Some of the uses of this compound are listed in this subsection.
- In concentrations between 2.5% to 10%, this compound can be applied on human skin to treat acne.
- Benzoyl peroxide can be used to remove dye stains and ink from many playthings such as vinyl dolls.
- It can also be used as a bleaching agent in cheese and bread.
- This compound is used in tooth whitening processes and also in cosmetic hair colouring.
- In the field of organic chemistry, benzoyl peroxide is a very convenient oxidant.
- The polymerization process of resins can be initiated with the help of this compound.
- It is also used as a catalyst in the synthesis of thermosetting polyester resins.
Frequently Asked Questions – FAQs
What is benzoyl peroxide good for?
How do you make benzoyl peroxide?
Which is best solvent for benzoyl peroxide?
What type of acid is benzoyl peroxide?
What are the chemical and physical properties of benzoyl peroxide?
Thus, the structure, properties, and uses of benzoyl peroxide are briefly discussed in this article. To learn more about this compound and other peroxides, such as hydrogen peroxide, register with BYJU’S and download the mobile application on your smartphone.

Comments