What are Mixtures?
Mixtures are formed when two or more substances (elements or compounds) mix together without participating in a chemical change. The substances need not necessarily mix in a definite ratio to form a mixture.
Some examples of mixtures include mixtures of sand and water, mixtures of sugar and salt, and mixtures of lime juice and water. There are two primary types of mixtures, namely homogeneous mixtures and heterogeneous mixtures.
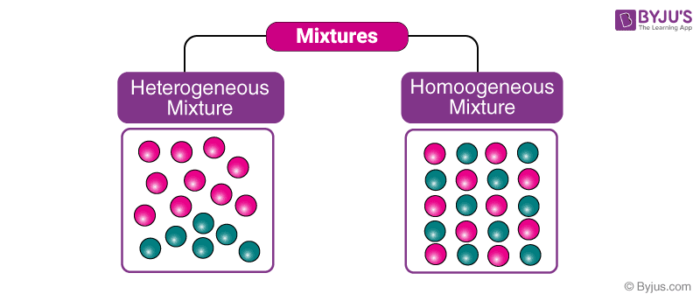
Heterogeneous and Homogeneous Mixtures
Table of Content
- Recommended Videos
- Heterogeneous and Homogeneous Definition
- Difference between Homogeneous and Heterogeneous Mixture
- Frequently Asked Questions – FAQs
Recommended Videos

Heterogeneous and Homogeneous Definition
What is a Homogeneous Mixture?
These are the types of mixtures in which the components mixed are uniformly distributed throughout the mixture. In other words, “they are uniform throughout”. We can observe only one phase of matter in a homogeneous mixture. Key points regarding such mixtures are:
- Particles are distributed uniformly
- We can’t judge a homogeneous mixture by just seeing it
- Homogeneous mixtures are also called solutions
- Uniform composition
- Example: rainwater, vinegar, etc.
What is a Heterogeneous Mixture?
This is a type of mixture in which all the components are completely mixed and all the particles can be seen under a microscope. We can easily identify the components and more than one phase can be seen by naked eyes.
Key points regarding this type of mixture:
- Particles are distributed non-uniformly
- We can judge a heterogeneous mixture by just seeing it
- Non-uniform composition
- Example: seawater, pizza, etc.
Difference between Homogeneous and Heterogeneous Mixture
Homogeneous mixture |
Heterogeneous mixture |
| It has a uniform composition | It has a non-uniform composition |
| It has only one phase | There are two or more phases |
| It can’t be separated out physically | It can be separated out physically |
| ‘homo’ means the same | ‘hetero’ means different |
| Example: a mixture of alcohol and water | Example: a mixture of sodium chloride and sand |
Example
Soft drink: Homogeneous or Heterogeneous mixture?
In a homogeneous mixture, all the components are uniformly distributed and in the soft drink, we find components like sweetener, carbon dioxide and water forming a single phase. Therefore, a soft drink is a homogeneous mixture.
Frequently Asked Questions – FAQs
What is a heterogeneous mixture?
A heterogeneous mixture is a mixture that is non-uniform and contains smaller component parts.
Which mixture is homogeneous?
A homogeneous mixture is a mixture throughout the solution in which the composition is uniform. The saltwater mentioned above is homogeneous due to the even distribution of the dissolved salt throughout the entire sample of saltwater.
What are heterogeneous and homogeneous mixture examples?
By combining two or more substances, a mixture is produced. A homogeneous solution tends to be identical, no matter how you sample it. Homogeneous mixtures are sources of water, saline solution, some alloys, and bitumen. Sand, oil and water, and chicken noodle soup are examples of heterogeneous mixtures.
Which best describes a heterogeneous mixture?
A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. By definition, a single-phase consists of a pure substance or a homogeneous mixture. There are two or more phases of a heterogeneous mixture.
Is air homogeneous or heterogeneous?
In air, all gases would have a uniform composition. Therefore, the air is an example of homogeneous mixture.
Stay tuned with BYJU’S to learn more interesting topics in Chemistry. Also, get various engaging and interactive video lessons to learn more effectively.
Read more:


Nicely explained and in a justified manner
Explained well.
Understood fully
Very very good app . It help me a lot in my studies . THANK YOU SO MUCH BYJU’S .
BYE
Good Explained viedo
nice video. I understood this topic. 👍
Nice explained
Good enough to study
Thanks for explaining 😊
All answers are well explaned
Very nicely explained in a very short manner And the video helped a lot too 👌🏻
The best explaination of the 2 mixtures so far. Well done. Thank you.
Thanks for this chapter explanation
Nice
Very very helpful 😊
Nicely derived
THANKS SIR FOR EXPLANING
Very much helpful
Even i am a member of byjus premium
Thanks very much for this wonderful note, you made me proud in front of my pupils while delivering this topic.
It is very very helpful for us sir👍🏻👍🏻💐💐
Nice explanation of mixture