What is Sublimation?
The term sublimation is the passage or the transformation or conversion that substances undergo when passing from one state to another, for example from a solid substance to gas.
Table of Contents
We can define sublimation as the transition of a substance from the solid phase to the gaseous phase without changing into the liquid phase. This process is an endothermic phase transition that occurs at a temperature and pressure below the triple point of the substance. Desublimation or deposition is the reverse of this process in which a gas is directly converted into solid-state.
-
-
-
- Elements and compounds mainly possess three different states at various temperatures.
- The transition from solid state to gaseous state requires a transition of solid-state to liquid state and liquid state to a gaseous state.
- If solids possess sufficient vapour pressure at a particular temperature then they can directly sublime into the air.
- Solids which have high pressure at their triple point show sublimation.
- The triple point is the point at which the pressure and temperature of the substance are such that it can exist in all three states of matter simultaneously. The triple point is a characteristic point of a substance.
- There are various examples of sublimation which are experienced by us in our everyday life.
-
-
Examples of Sublimation
-
-
-
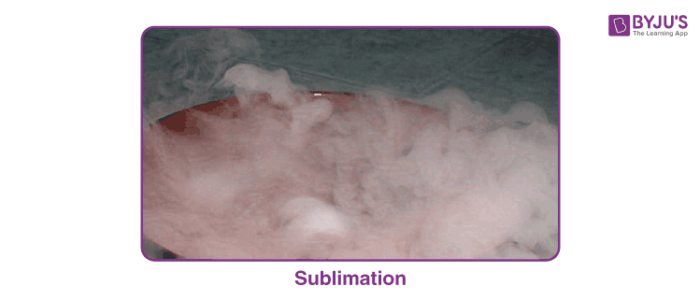
- The best example of sublimation is dry ice which is a frozen form of carbon dioxide. When dry ice gets exposed to air, dry ice directly changes its phase from solid-state to gaseous state which is visible as fog. Frozen carbon dioxide in its gaseous state is more stable than in its solid-state.
- Another well-known example of sublimation is naphthalene which is an organic compound. Naphthalene is usually found in pesticides such as mothballs. This organic compound sublimes due to the presence of non-polar molecules that are held by Van Der Waals intermolecular forces. At a temperature of 176°F naphthalene sublimes to form vapours. It desublimates at cool surfaces to form needle-like crystals.
-
-

Sublimation finds practical application in forensic sciences. Dye-sublimation printers help in rendering digital pictures in a detailed and realistic fashion which helps in the analysis of substances. Chemists usually prefer sublimation as a purification method to purify volatile compounds.
Recommended Videos

FAQs
1. What is the sublimation process?
Ans: Sublimation, including melting, freezing, and evaporation, is a form of phase transition, or shift in a state of matter. A substance transforms from a solid to a gas by sublimation without ever going through a liquid phase. A common example of sublimation is the dry ice, heavy CO2.
2. How is sublimation used in everyday life?
Ans: The bathroom air fresheners used. The strong sublimes and releases over a certain period of time the good smell in the bathroom. Naphthalene mothballs are used to scare moths and other pests home.
3. What things can sublimate?
Ans: A range of solids, including water, iodine, arsenic, and solid carbon dioxide (dry ice), can sublimate at normal temperatures and pressures. Other materials can sometimes be made to sublimate by creating conditions of low pressure.
4. What is the sublimation method of separation?
Ans: A mixture of solids is isolated by sublimation, one of which is sublime. Many liquids change immediately, without going through the liquid state, from solid to vapour on heating. This process is referred to as sublimation.
5. Does sublimation require heat?
Ans: The sublimation molar heat (or enthalpy) is the amount of energy that must be applied to a solid mole at constant pressure to convert it directly into a gas (without going through the liquid phase).
Join BYJU’S to fall in love with chemistry and download BYJU’S – The Learning App for any further support.

Comments