What is Dichromate?
Dichromate is an anion with the chemical formula Cr2O72-. It is used as a strong oxidising agent in organic chemistry as well as a primary standard solution in volumetric analysis. The chromate ion and dichromate ions are interconvertible in aqueous solution. The most common compound known is potassium dichromate which is an orange crystalline solid which readily decomposes to give potassium chromate and chromic oxide.
| Cr2O2−7 | Dichromate |
| Density | 2.68 g/cm³ |
| Molecular Weight/ Molar Mass | 294.185 g/mol |
| Boiling Point | 500 °C |
| Melting Point | 398 °C |
| Chemical Formula | Cr2O72- |
Dichromate Structure – Cr2O72-
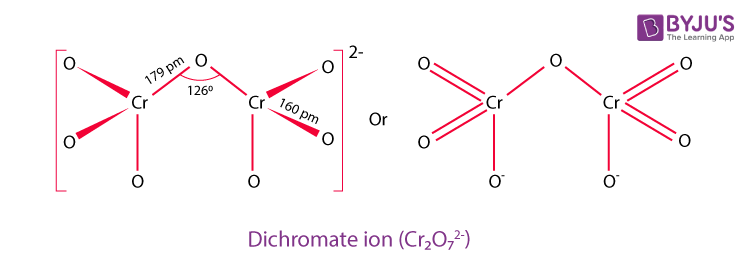
Physical Properties of Dichromate – Cr2O72-
| Odour | odourless |
| Appearance | Red-orange crystalline solid |
| Valency | 2 |
| pH | 4 |
| Oxidation state | +6 |
| Solubility | Moderately soluble in cold water and appreciably soluble in hot water. |
Chemical Properties of Dichromate – Cr2O72-
-
- Acidified solution of dichromate forms a deep blue colour with peroxide due to the formation of [CrO(O2)2].
Cr2O72- + 4H2O2 + 2H+ → 2CrO5 + 5H2O
-
- It reacts with hydrogen sulphide and oxidises it to sulphur, similarly it oxidises sulphites to sulphates, chlorides to chlorine, nitrites to nitrates, thiosulphates to sulphates and sulphur to stannic salts.
Cr2O72- + 3H2S + 8H+ → 2Cr3+ + 3S + 7H2O
Uses of Dichromate – Cr2O72-
- Used in photography for hardening of gelatin film.
- Used in chrome tanning in leather industry. It is also used in dyeing and provides Cr(OH)3 as a moderent.
- Used in volumetric estimation of ferrous salts, iodine and sulphites.
- Used in the preparation of other chromium compounds such as chrome alum, chrome yellow and chrome red.
Frequently Asked Questions
What are the uses of dichromate compounds?
Dichromate compounds have many applications. They are used as oxidizing agents and are also used in the preparation of various items such as waxes, paints, glues etc. However, potassium dichromate is carcinogenic and extremely toxic since it is a hexavalent chromium product.
What is the dichromate test?
Potassium dichromate paper can be used to check for the presence of sulfur dioxide since it transforms distinctly from orange to white. This is typical of all redox reactions in which hexavalent chromium is reduced to trivalent chrome.
Is sodium dichromate an oxidizing agent?
Sodium dichromate is a very powerful oxidant. Sodium dichromate in sulfuric acid is used for oxidizing primary alcohols but it is severely limited to the corresponding acid by overoxidation, through the aldehyde hydrate.


Comments