Chromium (VI) oxide, also known as chromium trioxide, is a chemical compound with a formula CrO3. It is a powerful oxidizer and a suspected carcinogen. It generally manufactured by treating sodium chromate with sulfuric acid. Annually, millions of grams of it are produced for the purpose of electroplating. In this short piece of article, learn more about the Chromium (VI) Oxide formula, its properties, chemical structure and uses.
Chromium (VI) Oxide Properties
| Properties of Chromium VI Oxide | |
| Name | Chromium (IV) Oxide |
| Also Known as | Chromium Trioxide, Chromic acid,
Chromic anhydride |
| Appearance and odour | Dark red granular solid and odourless |
| Chemical Formula | CrO3 |
| Melting Point | 197 °C |
| Boiling Point | 250 °C |
| Density | 2.7 g/cm3 |
| Molar Mass | 99.993 g/mol |
| Solubility in Water | Soluble |
Chromium (VI) Oxide Structure
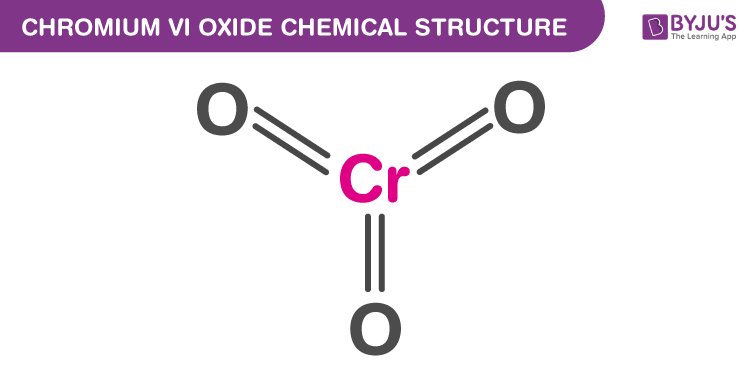
Chromium (VI) Oxide Uses
- Used in chrome plating
- Used in the production of synthetic rubies
- Used in aerospace applications
Safety Measurements
- Chromium trioxide is carcinogenic, toxic and highly corrosive in nature.
- Chromium trioxide is a strong oxidizer in nature ignite alcohols like organic compounds on contact.
Stay tuned to BYJU’S to learn more formulas of various other chemical compounds.

Comments