Table of Content
What are Transition Elements?Electronic Configuration of Transition ElementsGeneral Properties of Transition ElementsAtomic Ionic RadiiIonization EnthalpyFrequently Asked Questions
What are Transition Elements?
Transition elements (also known as transition metals) are elements that have partially filled d orbitals. IUPAC defines transition elements as an element having a d subshell that is partially filled with electrons, or an element that has the ability to form stable cations with an incompletely filled d orbital.
In general, any element which corresponds to the d-block of the modern periodic table (which consists of groups 3-12) is considered to be a transition element. Even the f-block elements comprising the lanthanides and the actinides can be considered as transition metals.
However, since the f-block elements have incompletely filled f-orbitals, they are often referred to as inner transition elements or inner transition metals. An illustration detailing the position of transition metals on the periodic table along with their general electronic configurations is provided below.
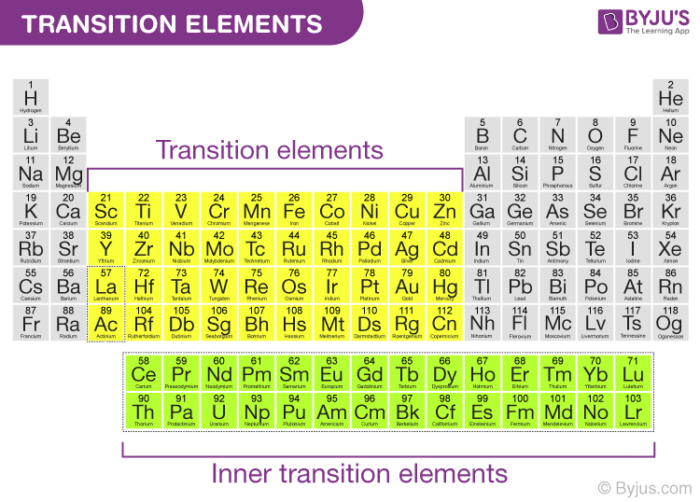
It is important to note that the element’s mercury, cadmium, and zinc are not considered transition elements because of their electronic configurations, which corresponds to (n-1)d10 ns2.
These elements have completely filled d orbitals in their ground states and even in some of their oxidation states. One such example is the +2 oxidation state of mercury, which corresponds to an electronic configuration of (n-1)d10.
Electronic Configuration of Transition Elements
The list of the first two rows of transition elements with their corresponding electronic configurations is tabulated below. It can be noted that in some of these elements, the configuration of electrons corresponds to (n-1)d5 ns1 or (n-1)d10 ns1. This is because of the stability provided by the half-filled or completely filled electron orbitals.
| Transition Elements | Atomic Number | Electronic Configuration |
| Sc | 21 | [Ar] 3d1 4s2 |
| Ti | 22 | [Ar] 3d2 4s2 |
| V | 23 | [Ar] 3d3 4s2 |
| Cr | 24 | [Ar] 3d5 4s1 |
| Mn | 25 | [Ar] 3d5 4s2 |
| Fe | 26 | [Ar] 3d6 4s2 |
| Co | 27 | [Ar] 3d7 4s2 |
| Ni | 28 | [Ar] 3d8 4s2 |
| Cu | 29 | [Ar] 3d10 4s1 |
| Zn | 30 | [Ar] 3d10 4s2 |
| Y | 39 | [Kr] 4d1 5s2 |
| Zr | 40 | [Kr] 4d2 5s2 |
| Nb | 41 | [Kr] 4d4 5s1 |
| Mo | 42 | [Kr] 4d5 5s1 |
| Tc | 43 | [Kr] 4d5 5s2 |
| Ru | 44 | [Kr] 4d7 5s1 |
| Rh | 45 | [Kr] 4d8 5s1 |
| Pd | 46 | [Kr] 4d10 |
| Ag | 47 | [Kr] 4d10 5s1 |
| Cd | 48 | [Kr] 4d10 5s2 |
It can be observed that the Aufbau principle is not followed by many transition elements like chromium. The reason for this is believed to be the relatively low energy gap between the 3d and 4s orbitals, and the 4d and 5s orbitals.
General Properties of Transition Elements
As discussed earlier, the elements zinc, cadmium, and mercury are not considered transition elements since their electronic configurations are different from other transition metals. However, the rest of the d-block elements are somewhat similar in properties and this similarity can be observed along each specific row of the periodic table. These properties of the transition elements are listed below.
- These elements form coloured compounds and ions. This colour is explained by the d-d transition of electrons.
- There is a relatively low gap in energy between the possible oxidation states of these elements. The transition elements, therefore, exhibit many oxidation states.
- Many paramagnetic compounds are formed by these elements, because of the unpaired electrons in the d orbital.
- A large variety of ligands can bind themselves to these elements. Due to this, a wide variety of stable complexes are formed by transition elements.
- These elements have a large ratio of charge to the radius.
- Transition metals tend to be hard and they have relatively high densities when compared to other elements.
- The boiling points and the melting points of these elements are high, due to the participation of the delocalized d electrons in metallic bonding.
- This metallic bonding of the delocalized d electrons also causes the transition elements to be good conductors of electricity.
Several transition metals have catalytic properties that are very useful in the industrial production of some chemicals. For example, iron is used as a catalyst in the Haber process of preparing ammonia. Similarly, vanadium pentoxide is used as a catalyst in the industrial production of sulfuric acid.
Atomic Ionic Radii
The atomic and ionic radii of the transition elements decrease from group 3 to group 6 due to the poor shielding offered by the small number of d-electrons. Those placed between groups 7 and 10 have somewhat similar atomic radii and those placed in groups 11 and 12 have larger radii. This is because the nuclear charge is balanced out by the electron-electron repulsions.
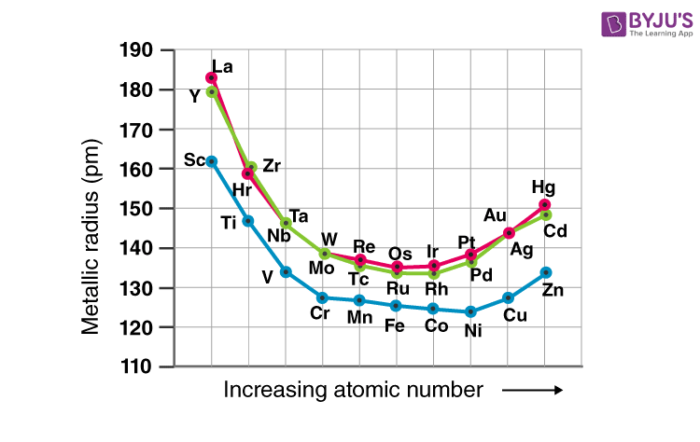
While traversing down the group, an increase in the atomic and ionic radii of the elements can be observed. This increase in the radius can be explained by the presence of a greater number of subshells.
Ionization Enthalpy
Ionization enthalpy refers to the amount of energy that must be supplied to an element for the removal of a valence electron. The greater the effective nuclear charge acting on the electrons, the greater the ionization potential of the element. This is why the ionization enthalpies of transition elements are generally greater than those of the s-block elements.
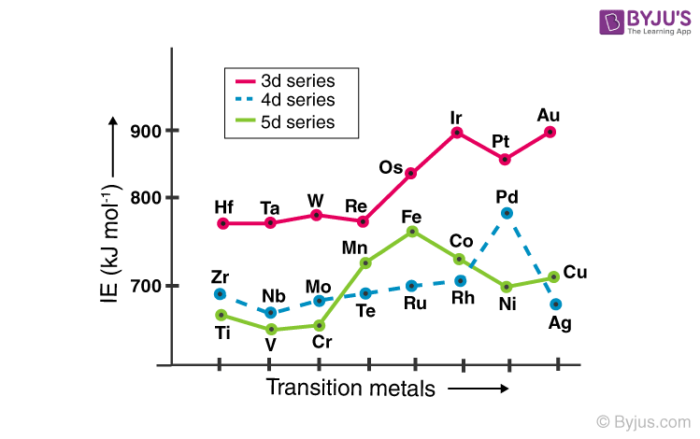
In a way, the ionization energy of an element is closely related to its atomic radius. Atoms with smaller radii tend to have greater ionization enthalpies than those with relatively larger radii. The ionization energies of the transition metals increase while moving along the row (due to the increase in atomic number).
Frequently Asked Questions
What are the General Characteristics of Transition Elements?
The d-block elements are known for their:
- Large charge: radius ratios
- High melting points and boiling points
- High densities and hardness.
- Formation of paramagnetic compounds
- Formation of coloured ions/compounds
- Ability to form stable complexes
These elements also exhibit a wide variety of oxidation states and tend to form compounds that act as catalysts in many chemical processes.
What are the Metallic Qualities of the Transition Metals?
The transition metals exhibit typical metallic properties such as malleability, ductility, high tensile strength, and metallic lustre. They are generally good conductors of heat and electricity and tend to crystallize in BCC (body-centred cubic), CCP (cubic close-packed), or HCP (hexagonally close-packed) structures.
However, trends can be observed in the metallic properties of the transition elements. For example, elements such as chromium and molybdenum are some of the hardest transition metals because they contain many unpaired electrons.
Explain the Reason Behind the High Melting/Boiling Points of Transition Elements.
The presence of unpaired electrons leads to the formation of metal-metal covalent bonds along with the metallic bonds. These strong bonds attribute high melting and boiling points to the elements. The presence of a partially filled d-orbital enables the transition elements to have a greater number of unpaired electrons, which in turn increases their ability to form covalent bonds along with metallic bonds.
For example, the elements with the greatest number of unpaired electrons (chromium, molybdenum, and tungsten) have the greatest melting and boiling points in their respective rows. On the other hand, metals such as zinc and mercury do not hold any unpaired electrons and hence have relatively low boiling and melting points.
Why are some Transition Metals Referred to as Noble Metals?
Some elements in the lower right corner of the d-block on the modern periodic table (such as gold, silver, and platinum) are often referred to as noble metals. These metals are highly unreactive owing to their low enthalpies of hydration and high ionization enthalpies.
These metals are highly resilient towards acids. However, metals like platinum, mercury, and gold can be dissolved in some acid mixtures such as aqua regia (a mixture of hydrochloric acid and nitric acid). It can be noted that silver does not dissolve in aqua regia.
What are the Uses of Transition Metals?
Iron, a transition metal, is widely used in the construction industry. It is usually alloyed into steel, which exhibits greater tensile strength and versatility. Iron is also used as a catalyst for the industrial production of ammonia via the Haber process. Titanium, another transition metal, is used in aircrafts, piping for nuclear power plants, and in artificial hip replacements.
The primary application of the transition element nickel is in the production of stainless steel. Copper, a transition metal, is widely used in electrical wiring because of its high tensile strength, malleability, ductility, and electrical conductivity.
Thus, the electronic configurations and the properties of the transition metals are briefly discussed in this article. To learn more about the transition elements and other groups of elements in the periodic table, register with BYJU’S and download the mobile application on your smartphone.


thank u so much I have learnt a lot and I have no doubt about this informtion
you should also provide us with some solved examples on this topic!!!
Click here to learn more about Transition Elements.