What is Polystyrene?
(C8H8)n is a synthetic aromatic hydrocarbon polymer with the chemical name Polystyrene.
Polystyrene is a hard, brilliantly transparent, stiff resin. It is produced by the polymerization of styrene and is the most widely used plastic. At room temperature, the thermoplastic polymer is a solid but when heated above 100 °C it flows. It becomes rigid again when it cools down. Polystyrene is insoluble in water.
Polystyrene is compound that is non-biodegradable with a couple of exceptions. It is easily dissolved by many aromatic hydrocarbon solvents and chlorinated solvents. It is widely used in the food-service industry as rigid trays, containers, disposable eating plates, bowls, etc.
Properties of Polystyrene – (C8H8)n
| (C8H8)n | Polystyrene |
| Molecular Weight/ Molar Mass | 104.1 g/mol |
| Density | 1.04 g/cm³ |
| Solubility in water | Insoluble |
| Melting Point | Approx 240 °C |
Polystyrene Structure ((C8H8)n Structure)
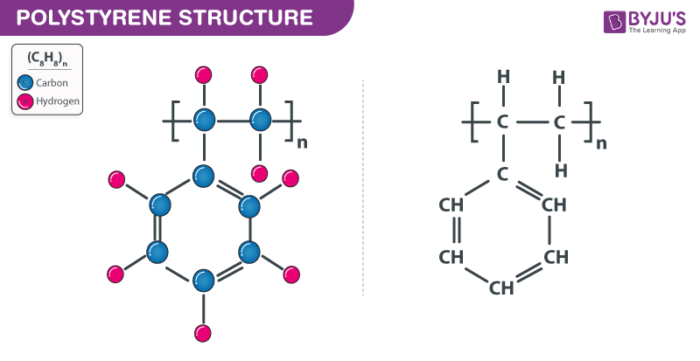
Polystyrene ((C8H8)n ) Uses
- Medically it is used for sterilizing test tubes, diagnostic components, and other medical devices.
- It is used to manufacture car parts which include knobs, instrument panels, sound dampening foam, etc.
- Polystyrene foodservice packaging keeps the food fresh for a longer period of time and is less expensive than alternatives.
- It is used in packaging consumer goods such as DVD cases, and egg cartons, to protect against spoilage or damage.
- It provides thermal insulation and is used in refrigerators, freezers, etc.
- Used in housing in all IT equipment such as Television, computer, etc.
Frequently Asked Questions
Is polystyrene plastic?
Polystyrene (PS) plastic is a thermoplastic that is naturally transparent and available both as a standard solid plastic and in the form of a rigid foam material. PS plastic is widely used in a number of consumer product applications and is also particularly useful for commercial packaging.
Where does polystyrene come from?
Polystyrene is one of the best-known synthetic polymers there are polyethene, polypropylene and polyester among others. Styrene, the liquid hydrocarbon from which EPF is made, was extracted from storax balsam in the late 19th century, which comes from a tree in Asia Minor called the Oriental sweet gum.
Is polystyrene toxic to humans?
Polystyrene contains the toxic substances Styrene and Benzene, possible carcinogens and neurotoxins that are harmful to humans. In fact, hot foods and liquids begin a partial breakdown of the Styrofoam allowing certain contaminants to be absorbed into our bloodstream and tissues.
Why is polystyrene useful?
Polystyrene is the packaging material of choice because it is light and cool. Snapping in half or collapsing is easy but, crucially, it is high in compression and thus protects fragile objects if dropped or crushed. Polystyrene is also a very strong insulator which means that it accumulates electrical charge easily.
Is polystyrene polar or non-polar?
Because polystyrene contains only carbon hydrogen bonds, it is non-polar and can dissolve only in non-polar solvents, much as dissolves. The general starch structure is depicted below. Starch contains bonds of oxygen carbon and oxygen hydrogen which make it a polar molecule.
Other related links:
| Classification Of Polymerization Reaction | Difference Between Thermoplastic And Thermosetting Plastic |

Comments