What is Hypochlorite?
Hypochlorite is an oxyacid of chlorine which contains monovalent chlorine that functions as a chlorinating agent or as an oxidizing agent.
The chemical formula of hypochlorite is ClO–. It is a conjugate base of HClO (hypochlorous acid).
Most of the hypochlorite salts are unstable in their pure form. They are usually handled as aqueous solutions. They are mainly used in bleaching and disinfection. They are also used as water treatment agents, in oxidation reactions, and chlorination.
Properties of Hypochlorite – ClO–
| Hypochlorite | ClO– |
| Molecular Weight of Hypochlorite | 51.449 g/mol |
| Solubility | Soluble in water |
| Conjugate acid | Hypochlorous acid |
| Boiling Point of Hypochlorite | Decomposes at 40 °C |
Structure of Hypochlorite (ClO–)
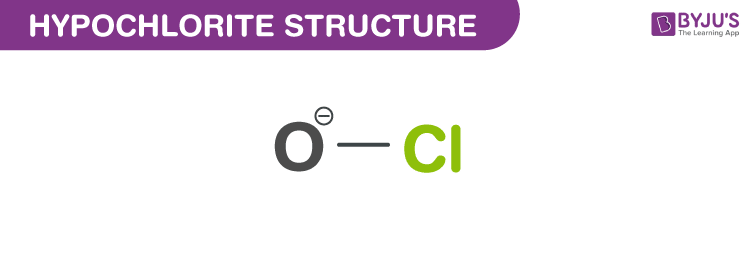
Structure of Hypochlorite – (ClO–)
Uses of Hypochlorite (ClO–)
- Hypochlorite is used as a chlorinating agent. This has the ability to chlorinate the electron-rich aromatic hydrocarbons.
- In organic chemistry, it is used to oxidize primary alcohols to carboxylic acids.
- It is a strong oxidizing agent and is used in Jacobsen epoxidation reaction to help in the conversion of Mn (III) to Mn (V).
- Sodium and calcium hypochlorite is used to whiten clothes.
- Domestically it is used to remove stains.
- Hypochlorites are used to lighten hair colour.
Health hazards
Hypochlorite is toxic when inhaled and ingested. Causes irritation to eyes, mucous membrane and the skin. When it comes in contact with organic materials, it may ignite. Its involvement in fire can enhance combustion or can also cause an explosion.
Frequently Asked Questions
What is hypochlorite solution used for?
Sodium hypochlorite (NaOCl) is a material that can be used effectively to cleanse water. It is used for surface purification, bleaching, odor elimination and the disinfection of water on a wide scale.
Is hypochlorite hazardous?
Hypochlorite can be used in whitening your whites, washing up the walls and serving as a perfect sanitizer. Yet bleach, (also known by its chemical name, sodium hypochlorite) can be very harmful if not properly treated.
What is the common name for sodium hypochlorite?
Sodium hypochlorite is a strong liquid oxidizing agent and has a greenish or yellowish hue. It is usually called bleach, because it is the active ingredient in bleach. The chemical formula is NaClO which consists of one atom of sodium (Na), one atom of chlorine (Cl) and one atom of oxygen (O).
Is sodium hypochlorite same as bleach?
Sodium hypochlorite is a solid white powder, its aqueous solution is most widely used. Sodium hypochlorite solutions is generally called bleach, though household bleach also contains small quantities of many other compounds including sodium hydroxide and calcium hypochlorite.
Is sodium hypochlorite carcinogenic?
The International Cancer Research Agency (IARC) has established that hypochlorite salts are not classifiable with respect to their human carcinogenicity.
Learn more about the physical and chemical properties of Hypochlorite (ClO–) from the experts at BYJU’S.


Comments