What is Atomic Mass?
Every particle of matter has some amount of mass associated with it whether small or large. Everything is made up of atoms. The mass of an atomic particle is called the atomic mass. This is commonly expressed as per the international agreement in terms of a unified atomic mass unit (amu).
The atomic mass of an atom is a feature that is closely related to its mass number. A single atom’s atomic mass is just its total mass, and it is commonly given in atomic mass units or amu. Carbon-12, an atom of carbon-containing six neutrons, has an atomic mass of 12 amu. Other atoms typically do not have round-number atomic masses. However, in general, an atom’s atomic mass will be very close to its mass number, with some variation in the decimal places.
Table of Contents
- Atomic Mass
- Atomic Mass of Elements
- What is Molecular Mass?
- Key Points
- Solved Example
- Frequently Asked Questions – FAQs
Atomic Mass
It can be best defined as 1/12 of the mass of a carbon-12 atom in its ground state. The mass of an atom can be accounted for by the sum of the mass of protons and neutrons which is almost equal to the atomic mass. This small change is due to the binding energy mass loss.
1 amu = 1.66 ×10−24 g
- The atomic weight of an atom is a dimensionless number when it is divided by unified atomic weight or Daltons.
- This is called the relative isotopic mass.
- The atomic masses of elements vary from 1.008 amu for hydrogen up to 250 amu for elements which have a very high atomic number.
- The mass of molecules can be determined by adding the average atomic mass of each atom in the molecule.
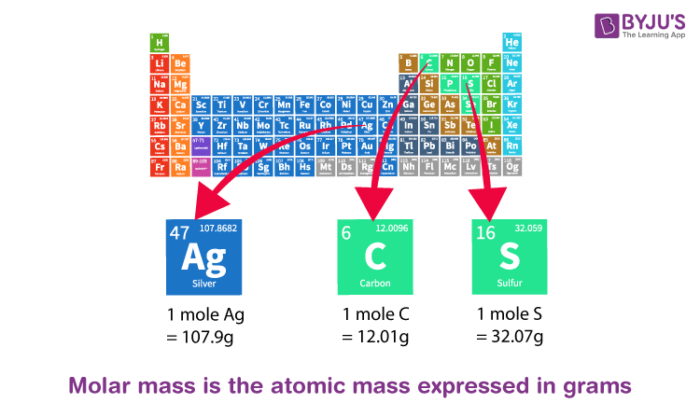
Atomic Mass of Elements
The Atomic mass of some elements is tabulated below.
| Atomic Mass of Iron | 55.845 u |
| Atomic Mass of Chlorine | 35.453 u |
| Atomic Mass of Sulphur | 32.065 u |
| Atomic Mass of Copper | 63.546 u |
| Atomic Mass of Potassium | 39.0983 u |
| Atomic Mass of Nitrogen | 14.0067 u |
| Atomic Mass of Calcium | 40.078 u |
| Atomic Mass of Phosphorus | 30.973762 u |
| Atomic Mass of Sodium | 22.989769 u |
What is Molecular Mass?
The molecular mass of a compound is the sum of the atomic masses of the atoms that form the compound.
Molecular mass is the amount of mass associated with a molecule. It is also called molecular weight. It can be calculated by adding the mass of each atom multiplied by the number of atoms of the element present in the molecule. For example, water is made up of 2 hydrogen atoms and 1 oxygen atom. The mass of a water molecule is equal to the average atomic mass of hydrogen multiplied by two plus the atomic mass of oxygen. The molecular mass of elements depends upon the constituent atoms of the molecule.
The molecular weight of molecules can be determined by the following methods:
- Mass spectrometry: This method is generally used in determining the mass of small molecules. This is reported as a monoisotopic mass.
- Hydrodynamic method: The weight is determined as per Mark-Houwink relations. This method requires calibration; hence it is also described as a relative molecular weight determination method.
- Static Light Scattering: Molecular weight is determined from the amount of light scattered using the Zimm method.
Key Points
- All matter is made up of atoms.
- Elements are substances made up of only one type of atom.
- Atoms have a central nucleus which contains protons and neutrons.
- Electrons move in orbits around the nucleus.
- Protons have a relative mass of 1 and a positive charge.
- Neutrons have a relative mass of 1 and are neutral.
- Electrons have negligible mass and a negative charge.
Recommended Videos
Introduction to Atomic and Molecular Weight

Solved Example
Question:
What is the molar mass of sulphur trioxide SO3, a molecular compound?
By definition, the molar mass of SO3 equals its molecular mass in grams. So the calculation of the molecular mass of SO3 is required first.
Solution:
The molecular mass (or formula mass) of a compound is the sum of the atomic masses of all the atoms in the molecule. The molecular mass of SO3 with 4 atoms in its molecule, is
1S = 1 x 32.07 amu = 32.07amu
3O = 3 x 16.00 amu = 48.00amu
The molecular mass of SO3 = 80.07 amu
Frequently Asked Questions – FAQs
Who discovered atomic mass?
John Dalton (between 1803 and 1805) and Jons Jacoband Berzelius (between 1808 and 1826) were the first scientists to measure atomic mass. The English chemist William Prout postulated an early atomic mass theory in a series of papers published in 1815 and 1816.
What is the simple definition of atomic mass?
An atomic mass (symbol: ma) is the mass of a chemical element’s single atom. It involves the masses of the three atomic subatomic particles: protons, neutrons and electrons.
Why atomic mass is important?
In chemistry, atomic mass is highly essential because it is the connection that we can measure in the laboratory between mass and moles, which are numbers of atoms. Most of what we are studying in chemistry depends on atom proportions.
What is the atomic mass number?
The total number of protons and neutrons (together known as nucleons) in an atomic nucleus is the mass number (symbol A, from the German word atomic weight, also known as the atomic mass number or nucleon number).
How do you find the molecular mass?
Molecular mass is a number equivalent to a molecule’s amount of nuclear masses. The molecular mass provides a molecule’s weight relative to that of the 12C atom, which is taken to have a weight of 12.
Is gold a heavy metal?
Gold, including its softness and malleability, is known as a heavy metal because each of its atoms is heavy on its own. That is a composite that is thick. Even among heavy metals, it is exceptional that many others neglect their brittleness of it.
What is the basic unit for mass?
In order to calculate the weight of an object, mass is used. For example, when you walk on a scale, you weigh the mass of your body. The most important mass units in the metric system of measuring are grams and kilograms.
Join BYJU’S to learn more about atomic weight and molecular masses.’


Thanks a lot