Ever wondered how forensic scientists analyze the smallest of ounces of evidence they find in a crime scene? Well, with the help of mass spectrometry you can even find out about the different isotopes associated with that element. Let us learn a bit more about Mass Spectrometry.

Mass Spectrometry
Mass spectrometry is an indispensable analytical tool in chemistry, biochemistry, pharmacy, medicine and many related fields of science. Mass spectrometry is employed to analyze combinatorial libraries, sequence biomolecules and help explore single cells or objects from outer space. Structure elucidation of unknown substances, environmental and forensic analytes, quality control of drugs, foods and polymers; they all rely to a great extent on mass spectrometry. Today mass spectrometry is interwoven with biology to an extent that basic considerations of proteomics research are dealt with in a MS journal.
Table of Contents
- What is Mass Spectroscopy?
- Mass Spectometry Detectors
- The Mass Analyzer
- What is a Quadrupole?
- Mass Spectroscopy Instrumentation
- How Does Mass Spectroscopy Work
- Applications of Mass Spectrometry
- Fields of Application of Mass Spectrometry
- Mass Spectrometry Advantages and Disadvantages
- Frequently Asked Questions – FAQs
What is Mass Spectrometry?
Mass spectrometry is an analytical method useful for calculating the mass-to-charge ratio ( m / z) of one or more molecules in the sample. Such measurements may also often be used to determine the precise molecular weight of the sample components. Mass spectrometry is an analytical method to find the molecular mass of a compound and indirectly helped to prove the identity of isotopes.
1. Principle of Mass Spectrometry
Based on Newton’s second law of motion and momentum, a mass spectrometer uses this property of matter to plot ions of varying masses on a mass spectrum. From the law, we infer how much mass is relevant to the inertia and acceleration of a body. This principle is applied to the aspect where ions with different mass to charge ratios are deflected by different angles in an electric or magnetic field.
2. Mass Spectrum
A mass spectrum is a graph obtained by performing mass spectrometry. It is a relation between the mass to charge ratio and ion signal.
3. Mass Spectrometry Diagram
- Inlet system
- Ionization
- Deflector
- Ion detector
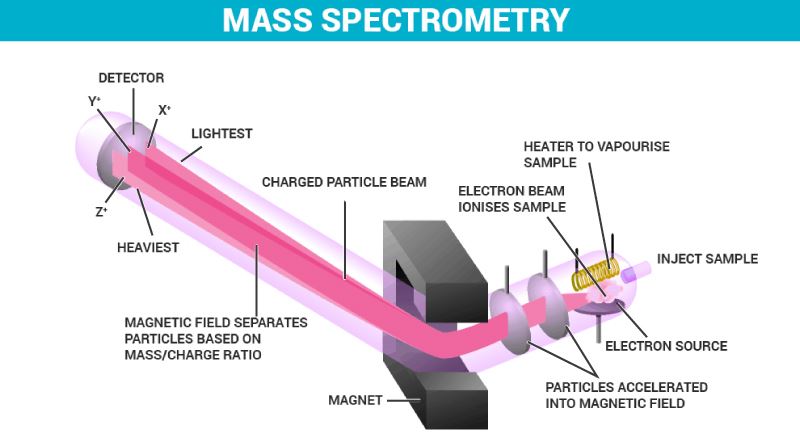
Mass Spectrometry Instrumentation
Mass Spectrometry Detectors
At different deflections a detector counts the number of ions. The data are plotted as a graph or continuum of various masses. Detectors function by recording the induced charge or current generated by an ion hitting or passing through a surface. Since the signal is very small it is possible to use an electron amplifier, Faraday cup, or ion-to-photon detector. To generate a spectrum the signal is greatly amplified.
The Mass Analyzer
When ionized, the ions are sorted and divided according to the mass-to – charge (m / z) ratio. A variety of mass analyzers are currently available, each of which has trade-offs related to speed of operation, separation resolution and other technical criteria. The different forms in use at the Broad Institute are described in the following section. The mass analyzer often works in concert with the ion detection system.
What is a Quadrupole?
Mass spectrometry determines the chemical by calculating the typical mass fragments formed by the ionization of the material. Test molecules are ionized by an electron beam, and the resulting molecular ion and component ions travel into a mass analyzer where their masses are measured.
Mass spectrometry is generally considered the benchmark for identification of unknown organic chemicals because it is highly sensitive and selective, and mass spectra are easily searchable against vast reference databases.
Mass Spectrometry Instrumentation:
The four main parts of mass spectrometry are discussed below:
- Ionizer – The bombarding of the sample is done by the electrons. These electrons move between cathode and anode. When the sample passes through the electron stream between the cathode and anode, electrons with high energy knock electrons out of the sample and form ions.
- Accelerator – The ions placed between a set of charged parallel plates get attracted to one plate and repel from the other plate. The acceleration speed can be controlled by adjusting the charge on the plates.
- Deflector – Magnetic field deflects ions based on its charge and mass. If an ion is heavy or has two or more positive charges, then it is least deflected. If an ion is light or has one positive charge, then it is deflected the most.
- Detector – The ions with correct charge and mass move to the detector. the ratio of mass to charge is analyzed through the ion that hits the detector.
How does Mass Spectrometry Work?
In a regular mass spectrometer, we initially have the material to be analyzed, but we need it to be ionized to pass through the spectrometer with enough energy. Thus, the sample is bombarded by electrons to ionize it.
This ionized beam is now passed through a series of electric or magnetic fields depending on the type of the sample and its properties. The ions are deflected by the field through which they are passed through in such a way that the ions with the same mass to signal ratio will follow the same path to the detector.
These charged and deflected ions are now incident onto a detector which is capable of distinguishing the charged particles falling on it. Based on the mass spectrum produced by the charged ions, we can identify the atoms or molecules constituting the sample by comparing them with known masses or through a characteristic fragmentation pattern.
Applications of Mass Spectrometry
Mass spectrometry is an efficient method to elucidate the chemical composition of a sample or molecule. More recently, it has been used to classify biological products, in particular proteins and protein complexes, in a number of species. Usually, mass spectrometers can be used to classify unknown substances by molecular weight measurement, to measure known compounds, and to determine the structure and chemical properties of molecules.
- Due to its capability to distinguish between substances, Mass spectrometry is used to determine unknown substances.
- To identify the isotopes of a substance.
- In analytical laboratories that study the chemical, physical and biological properties of substances. It is favored over several other analytical techniques as it has less background interference since it is performed in a vacuum.
Fields of Application of Mass Spectrometry
| Key application and field of application | Explanation |
| Elemental and isotopic analysis.
Physics Radiochemistry Geochemistry |
Elemental identification and isotopic abundance measurement of both short lived and stable species in physical and radioactivity in geochemistry and more recently in the life sciences. |
| Organic and bio-organic analysis
Organic chemistry Polymer chemistry Biochemistry and medicine |
Identification and structural characterization of molecules from small to very large as provided either by chemistry, physiological processes or polymer chemistry. |
| Structural elucidation
Organic chemistry Polymer chemistry Biochemistry and medicine |
Mass spectrometric experiments can be arranged consecutively to study mass selected ions in tandem mass spectrometry. Eventually products are subjected to a third level and so fourth. |
| Characterization of ionic species and chemical reactions
Physical chemistry Thermochemistry |
Tandem MS provides an elegant means for the study of unimolecular or bimolecular reactions of gas phase ions and for the determination of ion energetics. |
| Mass Spectral Imaging
Biomedical studies Material sciences |
Mass spectra can be obtained from micrometer sized areas on surfaces, translating the lateral distribution of compounds on surfaces into images, which in turn can be correlated to optical images. |
Mass Spectrometry Advantages and Disadvantages
Mass spectrometry is used for both qualitative and quantitative study of chemical substances. These can be used to classify a sample’s elements and isotopes, to determine molecular masses, and as a tool for helping to classify chemical structures. This can calculate the purity of the samples and the molar mass.
A big advantage of mass spec is that it is incredibly sensitive (parts per million) over many other techniques. It is an excellent tool for identifying or confirming the presence of unknown components in a sample. The disadvantages of mass spec are that identifying hydrocarbons that produce similar ions is not very good and it is not able to separate optical and geometric isomers. The disadvantages are offset by combining MS with other methods , for example gas chromatography. One disadvantage of mass spectrometry is over hydrocarbons. The method fails to distinguish between hydrocarbons producing similarly fragmented ions.
Thus, here we have seen a brief description of what a mass spectrometer is, its structure and how it functions.
Frequently Asked Questions – FAQs
What is the basic principle of mass spectrometry?
A mass spectrometer generates multiple ions from the sample being investigated, then separates them by their specific mass-to-charge ratio (m/z) and then records the relative abundance of each type of ion.
What are the applications of mass spectrometry?
Mass spectrometry is a powerful technique with many different applications in biology, chemistry, and physics, but also in clinical medicine and even in space exploration. It is used by separating molecular ions on the basis of their mass and charge to determine the molecular weight of compounds.
What is mass accuracy in mass spectrometry?
Mass precision is a metric that defines the difference between an ion’s measured mass/charge (m/Q) and that ion’s true, exact m/Q. The DAQ records mass accuracy in parts per million (ppm) for each of the three input calibration ions.
Is mass spectrometry quantitative or qualitative?
Design and application of mass spectrometry in biological fluids, tissues, species or cells for the qualitative and quantitative study of low molecular compounds and macromolecules.
What is unit mass resolution?
Unit resolution means that the next integer mass can be separated from each volume. That is, mass 50 can be distinguished from mass 51, and mass 1000 from mass 1001 can be distinguished. This concept is widely used to describe resolution on mass spectrometers of quadrupole and ion trap.
Why is mass spectrometry important?
Mass spectrometry is a way to calculate the mass of ions — electrically charged particles, atoms or molecules formed from them. This is used to describe the basic atomic and molecular processes, and also those of immediate interest to cell events.
How is mass spectrometry used in medicine?
Unit resolution means that the next integer mass can be separated from each volume. That is, mass 50 can be distinguished from mass 51, and mass 1000 from mass 1001 can be distinguished. This concept is widely used to describe resolution on mass spectrometers of quadrupole and ion trap.
What tool do mass spectrometry use?
A spectrometer is a tool designed to test light wavelengths over a broad range of the electromagnetic spectrum. This is commonly used for study of sample materials by spectroscopy. The incident light from the source of light may be emitted, absorbed or reflected through the sample.
What is positive and negative mode in Mass Spectrometry?
In the positive mode of ions, analyte molecules generally observed in the mass spectra were protonated and/or alkali adducted. The operating peaks corresponding to deprotonated analyte molecules are observed in the negative ion mode. ESI enables multiply charged ion output.
How is the mass spectroscopy graph plotted?
Mass Spectrometry is a process which determines the atomic mass of the atoms or molecules. It can be used to measure relative isotopic concentration, atomic and molecular mass, and the compound structure. The product of a Mass Spectrometry is a graph that plots mass against relative abundance per charge.
Which radiation is used in mass spectroscopy?
Photoionization can be used in experiments that seek to use mass spectrometry as a means of solving processes of chemical kinetics and branching of isomeric materials. In such cases a high-energy photon, either X-ray or uv, is used to dissociate stable gaseous molecules from He or Ar carrier gas.
To learn more in detail about mass spectrometry and other topics of chemistry, register with BYJU’S and download our app.
Read more:

Comments