What is a Battery?
A Battery is a device consisting of one or more electrical cells that convert chemical energy into electrical energy. Every battery is basically a galvanic cell where redox reactions take place between two electrodes which act as the source of the chemical energy.

Battery types
Batteries can be broadly divided into two major types.
- Primary Cell / Primary battery
- Secondary Cell / Secondary battery
Based on the application of the battery, they can be classified again. They are:
-
Household Batteries
These are the types of batteries which are more likely to be known to the common man. They find uses in a wide range of household appliances (such as torches, clocks, and cameras). These batteries can be further classified into two subcategories:
- Rechargeable batteries Nickel
Examples: Cadmium batteries, Lithium-Ion - Non-rechargeable batteries
Examples: Silver oxide, Alkaline & carbon zinc
- Rechargeable batteries Nickel
-
Industrial Batteries
These batteries are built to serve heavy-duty requirements. Some of their applications include railroad, backup power and more for big companies. Some examples are:
Nickel Iron
Wet Nickel Cadmium (NiCd) -
Vehicle Batteries
These are more user-friendly and a less complicated version of the industrial batteries. They are specifically designed to power cars, motorcycles, boats & other vehicles. An important example of a vehicle battery is the Lead-acid battery.
Primary Cell
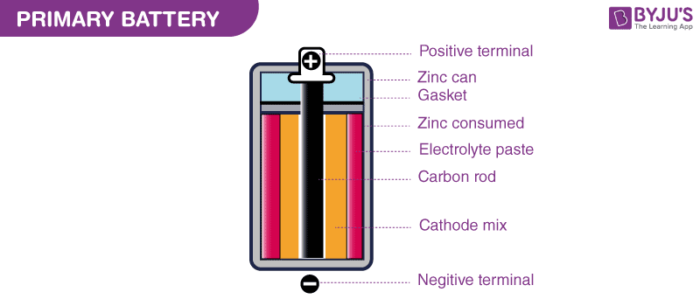
These are batteries where the redox reactions proceed in only one direction. The reactants in these batteries are consumed after a certain period of time, rendering them dead. A primary battery cannot be used once the chemicals inside it are exhausted.
An example of a primary battery is the dry cell – the household battery that commonly used to power TV remotes, clocks, and other devices. In such cells, a zinc container acts as the anode and a carbon rod acts as the cathode. A powdered mixture of manganese dioxide and carbon is placed around the cathode. The space left in between the container and the rod are filled with a moist paste of ammonium chloride and zinc chloride.
The redox reaction that takes place in these cells is:
At Anode
Zn(s) –> Zn2+ (aq) + 2e–
At Cathode
2e– + 2 NH4+ (aq) –> 2 NH3 (g) + H2 (g)
2 NH3 (g) +Zn2+ (aq) –> [Zn (NH3)2] 2+ (aq)
H2 (g) + 2 MnO2 (S) –> Mn2O3 (S) + H2O (l)
Thus, the overall cell equation is:
Zn(s) + 2 NH4+ (aq) + 2 MnO2 (S) –> [Zn(NH3)2] 2+ (aq) + Mn2O3 (S) + H2O (l)
Another example of the primary cell is the mercury cell, where a zinc-mercury amalgam is used as an anode and carbon is used as a cathode. A paste of HgO is used as an electrolyte. These cells are used only in devices that require a relatively low supply of electric current (such as hearing aids and watches).
Secondary Cell
These are batteries that can be recharged after use by passing current through the electrodes in the opposite direction, i.e. from the negative terminal to the positive terminal.
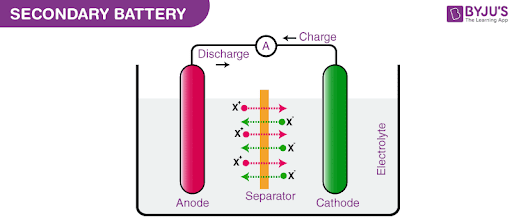
For example, a lead storage battery that is used in automobiles and inverters can be recharged a limited number of times. The lead storage battery consists of a lead anode and the cathode is a lead grid packed with lead dioxide. Sulphuric acid with a concentration of 38% is used as an electrolyte. The oxidation and reduction reactions involved in this process are listed below.
At Anode
Pb –> Pb2++ 2 e–
Pb+ SO42– –>PbSO4(electrode) + 2 e–
At Cathode
2 e–+ PbO2+ 4 H+ –> Pb2++ 2 H2O
2 e–+ PbO2+ 4 H++ SO42- –> PbSO4(electrode) + 2 H2O
In order to recharge these batteries, the charge is transferred in the opposite direction and the reaction is reversed, thus converting PbSO4 back to Pb and PbO2.
Another example of the secondary cell is the nickel-cadmium cell. These cells have high storage capacities and their lifespan is relatively long (compared to other secondary cells). However, they are difficult to manufacture and maintain.
To learn more about batteries and other related topics, register with BYJU’S and download the App.
Read more:


Good learning app