What is Copper(I) Oxide?
Copper(I) Oxide is also called as cuprous oxide, an inorganic compound with the chemical formula Cu2O. It is covalent in nature. Copper(I) oxide crystallizes in a cubic structure. It is easily reduced by hydrogen when heated. It undergoes disproportionation in acid solutions producing copper(II) ions and copper. When the cupric oxide is gently heated with metallic copper, it is converted into cuprous oxide. It acts as a good corrosion resistance, due to reactions at the surface between the copper and the oxygen in air to give a thin protective oxide layer.
Other names – Dicopper oxide, Red copper oxide, Cuprous oxide
| Cu2O | Copper(I) Oxide |
| Density | 6 g/cm³ |
| Molecular Weight/ Molar Mass | 143.09 g/mol |
| Boiling Point | 1,800 °C |
| Melting Point | 1,232 °C |
| Chemical Formula | Cu2O |
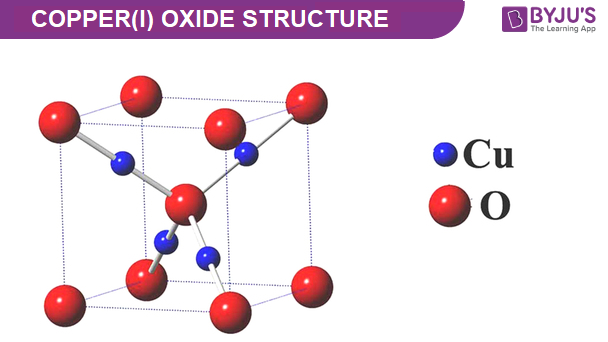
Copper(I) Oxide Structure – Cu2O
Physical Properties of Copper(I) Oxide – Cu2O
| Odour | No odour |
| Appearance | Red-coloured solid |
| Covalently-Bonded Unit | 3 |
| Heavy Atom Count | 3 |
| Complexity | 2.8 |
| Solubility | Insoluble in water |
Chemical Properties of Copper(I) Oxide – Cu2O
-
- Copper(I) oxide reacts with water in the presence of oxygen, forms copper(II) hydroxide. The chemical equation is given below.
2Cu2O + 4H2O + O2 → 4Cu(OH)2
-
- Copper(I) oxide reacts with hydrogen chloride forms Copper(I) chloride and water. The chemical equation is given below.
Cu2O + 2HCl → 2CuCl + H2O
Uses of Copper Oxide – Cu2O
- Used in antifouling paints for boat and ship bottoms; it is an effective control over corrosion.
- Used in paints for glass and porcelain.
- Used as a p-type semiconductor material that was used to make photocells for light meters and fabricate rectifiers.
- Used as a fungicide and seed dressing.
Frequently Asked Questions
What are the uses of cuprous oxide?
Cuprous oxide is widely used in marine paints as a dye, fungicide and an antifouling agent. Industrially, rectifier diodes based on this material were used as early as 1924, long before silicon became the standard.
What is the difference between CuO and Cu2O?
Cu2O is obtained by oxidizing copper metal or reducing sulfur oxide copper(II) solutions, while CuO is obtained by pyrometallurgical methods used to remove copper from ores. Most of the preservatives in wood are made from copper. This is often used as a pigment to shape various glazes.
Comment on the solubility of cuprous oxide in water
Cuprous oxide is practically insoluble in water and also in organic solvents. However, this compound is known to be soluble in aqueous solutions of ammonia and also in aqueous solutions of ammonium salts.


Comments