What is Conformation?
In alkanes, the distribution of electrons in a sigma molecular orbital is symmetrical around the internuclear axis of the C-C bond. Thus, it permits the possibility of free rotation about the C-C single bond. Due to this rotation, different spatial arrangements of carbon atoms in space are observed which can change into one another. Such spatial arrangement of carbon and hydrogen atoms which can be converted into one another by rotation around a C-C single bond is called conformation or conformer or rotamer. Alkanes can thus have an infinite number of conformations by rotation around C-C single bonds. However, this rotation is not completely free due to repulsive interactions between the electron clouds of C-H bonds. This repulsive interaction is termed as torsional strain.
Table of Contents
Conformational Isomers
Let us understand the fundamentals of conformation with the example of ethane. If we observe the ball and stick model of ethane and rotate one carbon atom keeping another carbon atom stationary about the C-C axis. We will observe that the rotations will result in an infinite number of spatial arrangements of hydrogen atoms attached to one carbon atom with respect to the hydrogen atoms attached to the other carbon atom. These different arrangements are better known as conformational isomers or conformers.
Predominantly, these can be broadly classified into two different cases:
-
-
-
-
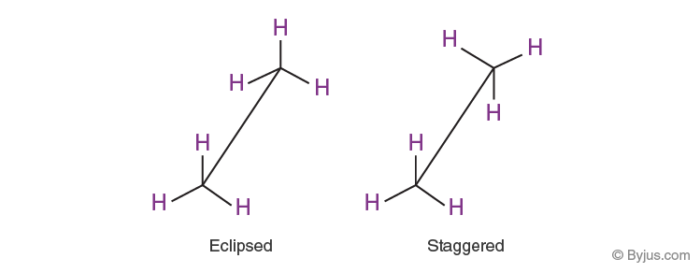
Eclipse conformation
Conformation in which hydrogen atoms are attached to two carbons stay nearest to each other as possible is known as eclipsed
-
Staggered conformation
Confirmation in which hydrogen atoms attached to two carbons are as far as possible with respect to each other is known as staggered The staggered conformation is thus relatively more stable in comparison to eclipse conformation as there are minimum repulsive forces, minimum energy due to many separations between the electron clouds of C-H bonds.
-
-
-
Representation of Eclipsed and Staggered Conformation:
Sawhorse projections:
In this kind of projection, the bond between carbon atoms is shown as a long straight line. The lower end of the line designates the front carbon atom whereas the upper end designates the rear carbon atom. Since each carbon atom in ethane is attached to three hydrogen atoms; each carbon atom has three lines attached designating C-H bonds inclined at an angle of 120° to each other.
Newman projections:
In this projection, out of the two carbon atoms present in ethane one which is nearer is shown as a dot whereas the rear carbon atom is represented as a circle. The three hydrogen atoms attached to each carbon atom are represented with the help of three lines either bulging out of the circle or diverging from the dotted lines. These lines are inclined to each other at an angle of 120° to each other.

Representation of Newman projection for conformation
Recommended Videos
Projection Formulae
Projection Formulae and Inter-conversion
Frequently Asked Questions – FAQs
What is the difference between conformational isomers and stereoisomers?
Stereoisomers: Two molecules with the same form but separate stereochemistry. Conformational isomers are briefly separate variants of the same molecule and are not known as isomers for this reason.
What are the three types of isomers?
Three types of structural isomers exist: chain isomers, functional group isomers, and positional isomers. These are structural isomers.
What is Newman projection formula?
The conformation of a chemical bond from front to back, with the front atom depicted as a dot and the back carbon as a circle, is visualized by a Newman projection, useful in alkane stereochemistry. The unique dihedral angle between the proximal and distal atoms is clearly demonstrated by this form of representation.
What is Diastereoisomerism?
Diastereomers are stereoisomers that can not be superimposed over each other and are not mirror images of each other. Diastereomers may be stereoisomers with two or three stereo centres. Diastereomers are stereoisomers that are not associated with artifacts and mirror images that are not enantiomers.
Why do diastereomers have different properties?
Diastereomers are stereoisomers that are not associated with artifacts and mirror images that are not enantiomers. Diastereomers may have varying reactivity and physical properties. They have multiple melting points and varying densities and boiling points. They have two stereo centres or more.
Still confused about conformation and conformational isomerism? Check out this video, which will definitely clear all your doubts on the topic from BYJU’S.
Read more:


Comments