Alkanes are the simplest hydrocarbons with all C-C bonds. Therefore, they are called saturated hydrocarbons. General formula for alkanes is CnH2n+2. All carbon atoms tend to complete their tetra valency by bonding with the same or different atoms. In alkanes, all carbon atoms form single covalent bonds with other carbon atoms.
Table of Content
The parent chain can be branched or unbranched and on the basis of that chemical and physical properties change. Alkanes are less reactive compared to other hydrocarbons like alkenes, alkynes etc. This is because, in alkanes, all carbon atoms are bonded with single covalent bonds which are strong and less reactive compared to double or triple covalent bonds of alkenes and alkynes respectively.
The members of the homologous series of alkanes are differed from each other by -CH2 unit. As the number of carbon atoms increases in the series, the molecular mass also increases.
What are Isomers?
Isomers are molecules with the same molecular formula but different structural formulas. This phenomenon is called as isomerism.
Due to different structure, isomers may have different chemical and physical properties also.
There are four carbon atoms in the given molecular formula. So these four carbon atoms can arrange in two different manners. They can either arrange in the straight chain of four carbon atoms or they can form a chain of 3 carbon atoms with one side chain.
| Structural formula | Name of isomer |
| CH3-CH2-CH2-CH3 | n-butane |
| CH3-CH(CH3)CH3 | 2-methylpropane or isobutane |
Recommended Videos
What are the Isomers of Butane?

Isomerism in Organic Compounds

How Many Isomers Does Butane have?
Butane is an alkane with four carbon atoms so molecular formula is C4H10. It has two isomers; n-butane and isobutane.
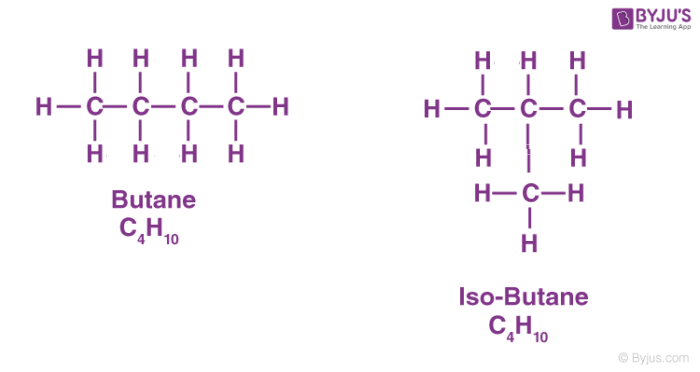
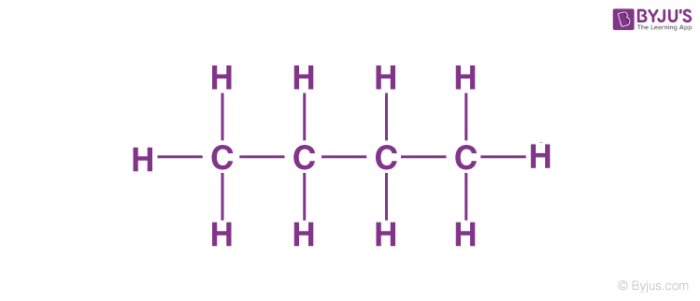
Here n-butane is a straight-chain compound with four carbon atoms bonded with single covalent bonds.
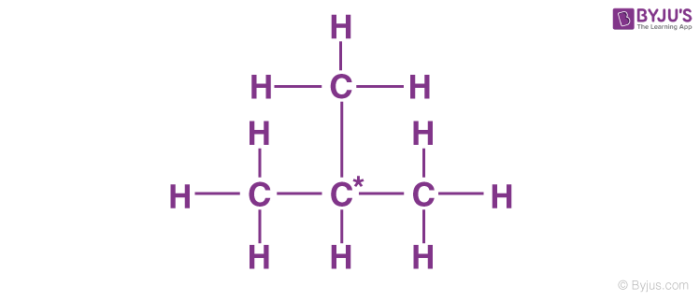
Another isomer is isobutane or 2-methylpropane in which three carbon atoms from the parent chain and one carbon atom is placed as the side chain at C-2 of the parent chain. All carbon atoms have 4 valencies which are satisfied either by carbon atoms or hydrogen atoms.
Constitutional Isomers of Butane
Constitutional isomers can be defined as the compounds that have the same molecular formula but different structural formulas. In other words, constitutional isomers have different connectivity of atoms in molecules. To determine that two molecules are constitutional isomers of each other or not, we have to count the number of each atom in both molecules.
The molecular formula must be the same for both the molecules but the arrangement of atoms should be different. For example, look at the structure of n-butane and isobutane.
Both of these molecules have the same molecular formula that is C4H10. The connectivity of atoms (carbon atoms). In the case of n-butane, all carbon atoms are in straight-chain whereas, in the case of isobutane, there is a side chain in the molecule. So they have different connectivity of atoms and are constitutional isomers of each other.
Conformational Isomers of Butane
- Stereoisomers which can be converted into one another by rotation around a single bond are called conformational isomers.
- Alkanes usually show conformational isomerism because of the presence of C-C bonds.
- For example, when we rotate the molecule of butane at the axis of C-C bond, we get eclipsed, gauche, and anti butane conformational isomers.
- Here eclipsed conformation has identical groups directly in-line with one another which makes it unstable.
- Gauche conformation stands for the presence of identical groups at 60 degrees from one another.
- Gauche is more stable compared to eclipse confirmation due to less steric hindrance between same molecules.
- In anti conformation, identical groups are 180 degrees from one another which makes it the most stable form.
- Each conformer is interconverted by rotating around the central carbon single bond like eclipsed conformer can convert into gauche conform by rotating 60 degrees.
- Rotation of eclipsed by 180-degree forms anti- conformer. Rotation of anti butane by 120 degrees forms gauche conformer.
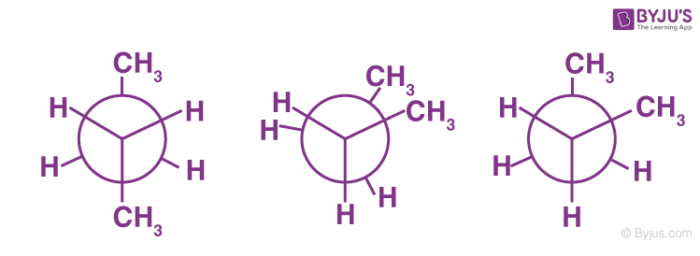
Staggered Conformation of Butane
- The study of conformers mainly involves the arrangement of atoms or groups with respect to central atom.
- There are different ways to represent conformers like Fisher projection, Saw-horse or Newman projection.
- Newman’s projection can be defined as the representation of a molecule in which the atoms and bonds are viewed along the axis of rotation.
- In a Newman projection, the front carbon is indicated as dot and back carbon atom as a circle. The substituent on carbon atoms can be viewed both in front of and behind the carbon-carbon bond.
- For example in ethane molecule, there are two possible conformations. One is with the dihedral angle 0° and another in which dihedral angle is 60 degrees.
- The first case is called as eclipsed conformation in which the H atoms of C-1 line up with the H-atoms of C-2.
- So we can say that in this conformation, the H-atoms are line up perfectly close to each other.
- In another conformer with dihedral 60 degrees, the H-atoms of C-1 is far enough to H-atoms of C-2. This is called the staggered conformation.
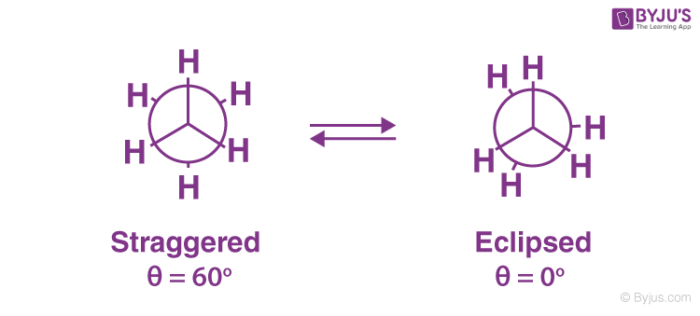
Since H-atoms are far enough in staggered conformer, it is more stable than eclipsed conformer. Staggered conformer of butane can exist in two forms; anti and gauche conformers. If we fix the position of one carbon atom of butane and rotate the other, it gives three types of conformers, eclipsed, anti and gauche.
There are two kinds of staggered conformations.
- In anti-form, both methyl groups of butane are at anti position. It is one of the most stable forms of butane.
- The other one is known as gauche or skew conformation.
Frequently Asked Questions – FAQs
What is isomerism?
Isomerism in organic chemistry is a phenomenon shown by two or more organic compounds having the same molecular formula but different properties due to differences in the arrangement of atoms along with the carbon skeleton (structural isomerism) or in space (Stereoisomerism)
What are the types of isomerism?
Two main forms of isomerism are structural or constitutional isomerism, in which bonds between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the relative positions of the atoms differ.
What is Newman’s projection formula?
The conformation of a chemical bond from front to back, with the front atom depicted as a dot and the back carbon as a circle, is visualized by a Newman projection, useful in alkane stereochemistry. The unique dihedral angle between the proximal and distal atoms is clearly demonstrated by this form of representation.
Define conformational isomers?
Stereoisomers which can be converted into one another by rotation around a single bond are called conformational isomers.
Why anti conformation is the most stable?
In anti conformation, identical groups are 180 degrees from one another which makes it the most stable form.

