Every spontaneous process leads to the formation of new products. Of all the processes known to us, some absorb energy while other result in evolution of energy. Hence, we always experience a change in enthalpy upon accomplishment of processes. This change in enthalpy can be due to the enthalpy of atomization, solution etc. Some common enthalpy changes are explained below:
Table of Contents
Enthalpy of Atomization:
In chemistry, the enthalpy of atomisation is the enthalpy change that accompanies the total separation of all atoms in a chemical substance. For example: atomization of methane molecule.
CH4 (g) → C (g) + 4H (g) ΔaH0= 1665.0 kJ mol-1
For diatomic molecules, the enthalpy of atomization is equal to the enthalpy of bond dissociation. For example: the atomization of dihydrogen molecule.
H2 (g) → 2H (g); ΔaH0= 435.0 kJ mol-1
Enthalpy of solution:
Enthalpy of the solution, ΔsolH0 is the enthalpy change when one mole of a substance is completely dissolved in a solvent. For example: enthalpy of dissolution of ionic compound in water.
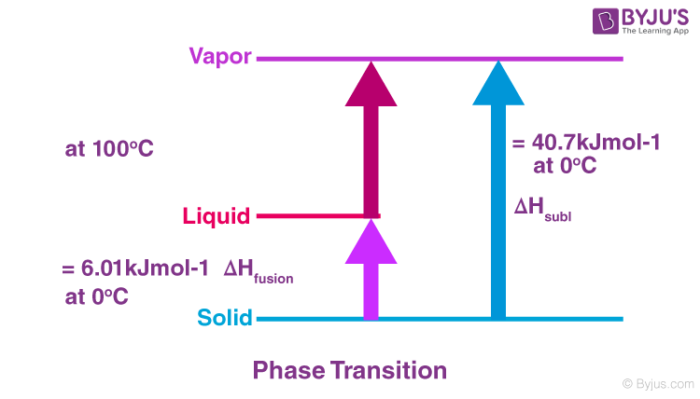
Enthalpy change during phase transition:
When a substance undergoes a phase transition, that is the phase of a substance changes from one form to another, some energy is released or absorbed. For example, when the ice melts to water, energy is required for melting. Common enthalpy change during phase transition includes:
Standard Enthalpy of vaporization:
The standard enthalpy of vaporization, ΔvapH0 is the amount of heat required to vaporize one mole of a liquid at constant temperature and under standard pressure (1bar).
Standard enthalpy of sublimation:
Standard enthalpy of sublimation, ΔsubH0 is the change in enthalpy when one mole of a solid substance sublimes at a constant temperature and under standard pressure (1bar).
Frequently Asked Questions – FAQs
How do you calculate atomisation energy?
If pressure is kept constant, the change in enthalpy is proportional to the change in a system’s internal energy. Therefore, the atomization enthalpy equals the sum of the fusion and vaporisation enthalpies.
Which element has the lowest enthalpy of atomisation in the series Sc (Z = 21) to Zn (Z = 30)?
Sc (Z = 21) to Zn (Z = 30) belong to the fourth period of the periodic table. The degree of metallic bonding an element undergoes influences the atomization enthalpy. The more extensive an element’s metallic bonding is, the more its atomization enthalpy would be. The presence of some unpaired electrons in all transition metals with the exception of Zn, explains why their metallic bonds are stronger. Zn has the lowest enthalpy of atomization because it lacks these unpaired electrons, which weakens the inter-atomic electrical connection.
What is mean by the enthalpy of atomisation?
Atomization enthalpy is the amount of modification in enthalpy as the bonds of a complex are dissolved and the constituent atoms are reduced to individual atoms. Ordinary atomization enthalpy is the transition of enthalpy as 1 mol of a material under standard conditions (298.15 K, 1 bar) is fully dissociated through atoms.
Why is enthalpy of atomisation endothermic?
The energy needed to detach one electron from each mole of free gaseous atoms of that element is the first ionisation energy of an atom. There are still positive (ie endothermic) atomization enthalpies and ionisation enthalpies.
Is Bond enthalpy the same as enthalpy of atomisation?
Atomization enthalpy (atH) is the transition in enthalpy when one mole of gaseous atoms is formed from an atomic matter. In the other hand, the energy needed to split one mole of bond to give separate atoms in the gaseous state is bond enthalpy / bond energy / bond dissociation enthalpy.
For detailed discussions on standard enthalpy of atomization, solution and phase transition, download BYJU’S – the learning app.

Excellent notes for chemistries