What is Sucrose (C12H22O11)?
Sucrose is a molecule composed of two monosaccharides, namely glucose and fructose. This non-reducing disaccharide has a chemical formula of C12H22O11.
Sucrose is commonly referred to as table sugar or cane sugar.
In a C12H22O11 molecule, the fructose and glucose molecules are connected via a glycosidic bond. This type of linking of two monosaccharides called glycosidic linkage. Sucrose has a monoclinic crystal structure and is quite soluble in water. It is characterized by its sweet taste.
William Miller, an English chemist, coined the word sucrose in the year 1857. It is widely used as a sweetener in food. C12H22O11 can be obtained from sugar beets or sugar canes, but it must be refined to be fit for human consumption. Refined sucrose (or sugar) is a popular ingredient in many food recipes because of its sweet taste.
Table of Content
- Structure of Sucrose
- Chemical Data of Sucrose
- Physical Properties of Sucrose
- Chemical Properties of Sucrose
- Uses of Sucrose
- FAQs
Structure of Sucrose
As discussed earlier, sucrose is a disaccharide which is made up of two monosaccharides. The structure of a sucrose molecule is illustrated below.
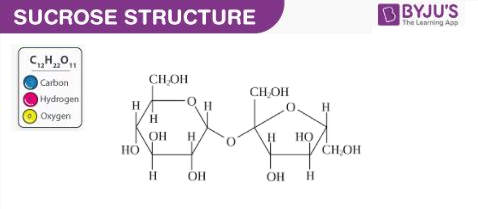
The glycosidic linkage that connects the two carbohydrate groups can be observed in the illustration provided above. There are no anomeric hydroxyl groups in a sucrose molecule. It can, therefore, be classified as a non-reducing sugar (since it does not act as a reducing agent).
Chemical Data of Sucrose
| Chemical Formula of Sucrose | C12H22O11 |
| Molar Mass or Molecular Weight | 342.30 g/mol |
| Density | 1.587 g/cm3 |
| Physical Appearance | White, crystalline solid |
| Melting Point | Decomposes at 459 K |
Physical Properties of Sucrose
- Sucrose has a monoclinic crystal structure.
- When subjected to high temperatures (over 186oC), this compound decomposes, yielding caramel.
- Its solubility in water at a temperature of 20oC is 203.9g/100mL
- The standard enthalpy of combustion corresponding to sucrose is 5647 kJ.mol-1.
Chemical Properties of Sucrose
- Sucrose can undergo a combustion reaction to yield carbon dioxide and water.
- When reacted with chloric acid, this compound yields hydrochloric acid, carbon dioxide, and water.
- Upon hydrolysis, the glycosidic bond linking the two carbohydrates in a C12H22O11 molecule is broken, yielding glucose and fructose.
- Sucrose can be dehydrated with the help of H2SO4 (which acts as a catalyst) to give rise to a black solid which is rich in carbon.
Thermal Degradation of Sucrose
When heated to temperatures above 186 degrees Celsius, sucrose undergoes a decomposition reaction to give rise to caramel. In a manner that is similar to other carbohydrates, sucrose undergoes combustion in the presence of oxygen to yield water and carbon dioxide as the products. It can also be noted that sucrose can be reacted with potassium nitrate (a powerful oxidizing agent with the chemical formula KNO3) to yield a special type of fuel known as rocket candy. The chemical equation for the reaction between sucrose and potassium nitrate is provided below.
C12H22O11 + 6KNO3 → 3K2CO3 + 3N2 + 9CO + 11H2O
It can also be noted that sucrose undergoes a combustion reaction with chloric acid to yield hydrochloric acid, water, and carbon dioxide. This reaction can be represented by the following chemical equation:
C12H22O11 + 8HClO3 → 8HCl + 11H2O + 12CO2
Dehydration of Sucrose with Sulfuric Acid
Sucrose can be subjected to dehydration in the presence of sulfuric acid in order to obtain a black solid that is rich in carbon. The idealized chemical equation for this process is provided below.
C12H22O11 + H2SO4 → 11H2O + 12C (carbon-rich solid) + heat
It can also be noted that small quantities of SO3 can be produced in this process.
Uses of Sucrose
Some of the important uses of this compound are listed below.
- Sucrose is one of the most important components of soft drinks and other beverages.
- This compound is used in many pharmaceutical products.
- It serves as a chemical intermediate for many emulsifying agents and detergents.
- It also serves as a food thickening agent and as a food stabilizer.
- The shelf lives of many food products, such as jams and jellies, are extended with the help of this compound.
- The use of sucrose in baking results in the brown colour of the baked products.
- This compound also serves as an antioxidant (a compound that inhibits oxidation).
- Sucrose is widely used as a food preservative.
Thus, the structure of a sucrose molecule, its physical and chemical properties, and its uses are discussed briefly in this article. To learn more about this disaccharide and other disaccharides, such as lactose, register with BYJU’S and download the mobile application on your smartphone.
Frequently Asked Questions-FAQs
1. What is sucrose made of?
Sucrose is a disaccharide sugar which means that it consists of two units of monosaccharide sugar. The two units are glucose and fructose, for sucrose. The name saccharose is derived from the French word fruit.
2. What is the use of sucrose?
Sucrose is used in foods and soft drinks as a sweetener, in syrup processing, in invert sugar, confectionery, preserves and jams, demulcent, medicinal products, and caramel. Sucrose is also a chemical carrier for detergents, emulsifiers, and other derivatives of saccharose.
3. What foods contain sucrose?
Sucrose is found in fruits and vegetables, and is processed for use in cooking and food processing from sugar cane and sugar beets. The sucrose found naturally in sugar cane, sugar beets, bananas, grapes, carrots, and other fruits and vegetables in your sugar bowl is the same sucrose.
4. Is sucrose soluble in ethanol?
Sugar or sucrose is only slightly soluble in ethanol. In addition, if the alcohol is cold it will dissolve even less of the sucrose. The sugar not dissolving within the ethanol settles at the bottom of the bottle. The salt is also very water-soluble.
5. What is the function of sucrose in plants?
Sucrose is the most common type of carbohydrate used for the carriage of carbon in a plant. Sucrose can be dissolved in water, thus retaining a stable structure. Sucrose will then be transported into the phloem by plant cells, the special vascular tissue intended for sugar transport.


really very useful for studies, I always visit this site to clear my doubts, really iseful