Magnesium Hydroxide formula is given and explained in this article along with its structural formula. Top recall about Magnesium hydroxide, it is a non-toxic inorganic base. It occurs naturally in brucite mineral and is commonly used as antacids.
Magnesium Hydroxide Chemical Formula
Magnesium hydroxide has a magnesium atom to which two hydroxyl groups (OH) are attached. Its molar mass is 58.32 g/mol and is an ionic compound. The chemical formula of magnesium hydroxide is given as the follows.
| Chemical Formula for Magnesium Hydroxide: MGOH2 |
Structural Formula of Magnesium Hydroxide
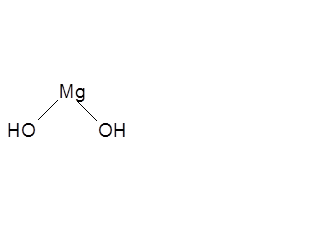
Magnesium hydroxide is non-toxic and by diluting it, a white milky suspension is formed, known as “milk of magnesia” which is used as an antacid. Apart from that, magnesium hydroxide is also used as an antiperspirant, laxative, fire retardant, to treat sores, etc.
To know the chemical formulas of various other compounds, stay tuned with BYJU’S and register now to get complete assistance for the exams. BYJU’S also provide notes, video lessons, and other study materials to help students learn more effectively.

Comments