A number of chemical reactions take place around us. We see a flaky brown-coloured layer appearing on the surface of iron articles such as gates and car bodies. During the festival of Diwali, we light crackers that render bright light and sound. The bright light results from the burning of components such as magnesium which are used as one of the components of the crackers and sparklers. Matter interacts to form new products through a process called a chemical reaction or chemical change. In this section, we will learn about these chemical reactions.
Types of Reactions
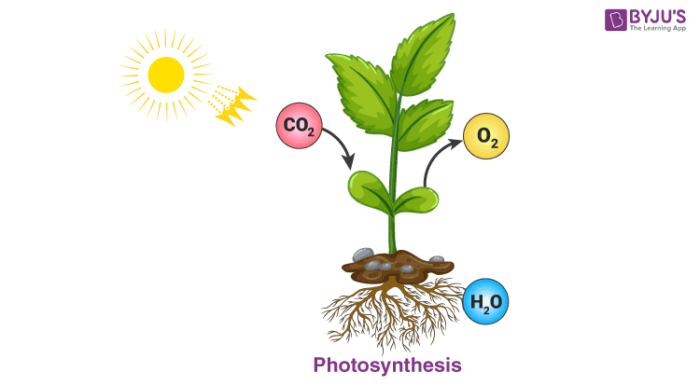
Photosynthesis: The process of photosynthesis by which autotrophs manufacture their food is another chemical reaction. In the presence of sunlight and chlorophyll, plants manufacture glucose from carbon dioxide and water from the environment. This process releases oxygen which is the essential element for us to survive on earth.
6CO2 (g)+6H2O (l) ———>C6H12O6 (aq) + 6O2 (g)
Rusting: The process of oxidation (reactions in the presence of oxygen) results in the formation of a brown flaky layer over the metal surfaces such as iron. This layer is formed due to the oxidation of the top layer to form the metal oxide which is known as rust. Similar layers are formed on other metals such as silver where a green-coloured layer is formed.
4Fe+3O2+xH2O————-> 2 Fe2O3.xH2O

Cellular Respiration: The process of respiration in humans also involves a chemical reaction. The glucose molecules undergo oxidation to produce carbon dioxide and water along with energy.
C6H12O6 (aq) + 6O2 (g) → 6CO2 (g) + 6H2O (l) + Energy
Anaerobic Respiration: When an object undergoes fermentation, the components that contain sugar or starch get converted to gases, acids, and alcohol.
C6H12O6 → 2 C2H5OH + 2 CO2
Combustion: The process of combustion involves the oxidation of a material in the presence of oxygen from the environment and heat to produce ash, smoke, and other gases.
C+O2 ———–> CO2
Acid-base reactions: The weak acids produced inside our mouth due to sugary foods and bacteria overnight are neutralized with the base present in the toothpaste we use every day.
Acid + Base → salt + water
Similarly, the oxide layer on metallic artefacts is removed with weak acids such as vinegar. This process renders the layer of the metal clean as a fresh layer of metal is exposed.
CH3COOH+FeO———>CH3COOFe+H2O

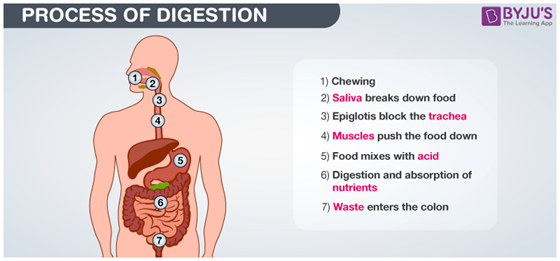
Digestion
Thousands of chemical reactions take .place during digestion. Enzyme amylase present in saliva breaks down sugars and carbohydrates present in food to form simpler forms which the body can absorb. Hydrochloric acid in the stomach reacts with food to further break it down. So during digestion, proteins break into amino acids, fats break into fatty acids and glycerol and carbohydrates break into simple sugars.

Comments