What is Citric Acid?
Citric Acid is a weak acid with a chemical formula C6H8O7. It can occur in two forms – monohydrate or water-free (anhydrous). This acid is usually found in citrus fruits like lemons, oranges etc. It is considered as a tribasic acid. It is odourless, sour in taste, and appears as a white crystalline solid. It has a monoclinic crystal structure. This organic acid was isolated for the first time by chemist Carl Wilhelm Scheele in the year 1784. Since it is similar in resemblance to table salt,it is sold in the market as sour salt.
Table of Contents
- Properties of Citric Acid – C6H8O7
- Citric Acid structure – C6H8O7
- Uses of Citric Acid
- Frequently Asked Questions
Properties of Citric Acid – C6H8O7
| C6H8O7 | Citric Acid |
| Molecular Weight/ Molar Mass | 192.124 g/mol |
| Density | 1.66 g/cm³ |
| Boiling Point | 310 °C |
| Melting Point | 153 °C |
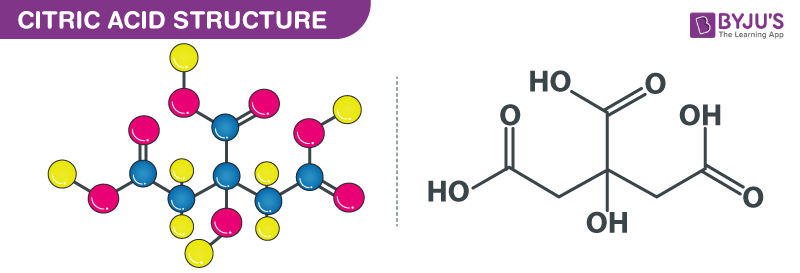
Citric Acid structure – C6H8O7
Uses of Citric Acid
- It is used as an antioxidant
- It is used as a cleaning agent – as an ingredient in kitchen and bathroom cleaning solution
- It is used as an emulsifying agent in ice creams
- It is used to add a sour taste to soft drinks and other food items
- It used in shampoo
- It is used in sucrose crystallization in caramel
- It is used in food colouring
- It is used as a natural preservative
- It is used to remove the chalky deposit from evaporators, kettles, boilers etc.
Frequently Asked Questions
What are the uses of citric acid?
Citric acid is an organic compound that is found in citrus fruits. It is a natural preservative and is also used in foods and soft drinks to imbue an acidic (or a sour) flavour. It is essential in biochemistry as an intermediate in the cycle of citric acid, and thus occurs in the metabolism of almost every living organism.
Is citric acid dangerous?
Naturally, citric acid is present in citrus fruits, but synthetic forms (generally made from a mold type) are widely added to foods, medications, supplements, and cleaners. While mold residues from the manufacturing process can in rare cases cause allergies, citric acid is generally considered to be a safe substance.
How to prepare a solution of citric acid?
Combine citric acid crystals (sometimes referred to as sour salt) with 1 or 2 pints of distilled boiling water per pound of citric acid to form the citric acid solution. Put the crystals of citric acid in a non-metal bowl and gradually pour the boiling water into the bowl, stirring with a non-metal spoon.
Learn more about the structure and properties of C6H8O7 from the expert faculties at BYJU’S.

Comments