Preparation of a True Solution of Common Salt, Sugar and Alum
- Solution: A solution is a combination of two or more substances that is homogenous in nature. Solid solutions, such as alloys, liquid solutions, such as lemonade, and gaseous solutions, such as air, are examples of possible solutions. Solute and solvent combine to form a solution.
- Solute: The solute is the part of the solution that is dissolved in the solvent.
- Solvent: Solvent is the component in a solution that dissolves the other components in it.
Aim:
To prepare a true solution of common salt, sugar and alum in water and distinguish between these on the basis of transparency, filtration criterion, and stability.
Theory:
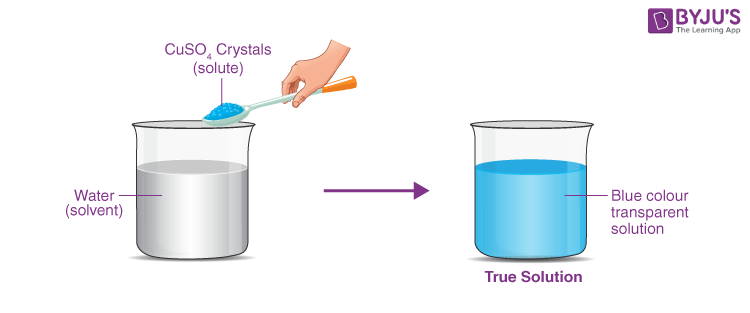
True solution is defined as a solution containing solute particles that are less than 1 nm (10-9 metres) in diameter and are invisible to the human eye. They don’t scatter a ray of light, the particles do not separate through filtration, and they do not settle down.
Properties of True Solutions:
- A true solution is a mixture of solute and solvent that is homogenous.
- Solute particles are less than 1 nm (1 nm =10-9m).
- The elements do not scatter light and do not exhibit the Tyndall effect.
- Filtration will not be able to separate the particles.
- The solution is stable (remains uniform).
- The solution is clear.
| Also Read: Preparation of a True Solution of Common Salt, Sugar and Alum Viva Questions |
Materials Required:
Beakers, Common salt (Sodium chloride), Sugar, Alum, Test tubes, Glass Rod, Water.
Procedure:
Step 1: Fill three beakers with 100 mL of water and label them A, B, and C, correspondingly.
Step 2: In each beaker, 10g of finely powdered common salt, sugar, and alum are added separately.
Step 3: Using the glass rod, stir the solution.
Step 4: Place the solutions in test tubes and mark them with the letters A, B, and C.
Observation Table:
| Property | Experimental Procedure | Observation | Inference |
|---|---|---|---|
| Transparency | Each test tube has a small strip of cellophane paper glued on it, and the coloured paper of each test tube can be seen from the other side. | Colour spot is evidently seen on test tubes when seen from the other side. | A true solution is transparent. |
| Filtration Criterion | Filtrate the contents of test tubes labelled A, B, and C. | There is no residue on the filter paper, and the filtrate is clear. | Filtration cannot separate solid particles from true solution. |
| Stability | Allow 20-25 minutes for the test tubes to rest without being disturbed. | There is no change in the solutions. | True solutions are stable and do not exhibit component deposition. |
Results and Discussion:
True solutions are clear and transparent. They pass through the filter paper without leaving any trace. The filtrate is translucent as well.
Precautions to be taken during the experiment:
(i) Handle the materials and solutions with care.
(ii) While filtering a solution, pour the contents into the funnel using a glass rod.
(iii) Do not disturb the sample during the stability test.
Viva Voce
1. Give one example of liquid solution.
Lemonade + water.
2. What is the size of particles in true solution?
The size is less than 1 nm (10-9 metre) in diameter.
3. Why does a true solution not scatter a beam of light?
It does not scatter a beam of light due to its small particle size.
4. What is concentration of solution?
The amount of solute present in a given solution.
5. Why is water called a universal solvent?
Water is referred to as a universal solvent since it dissolves the maximum number of substances.

Comments