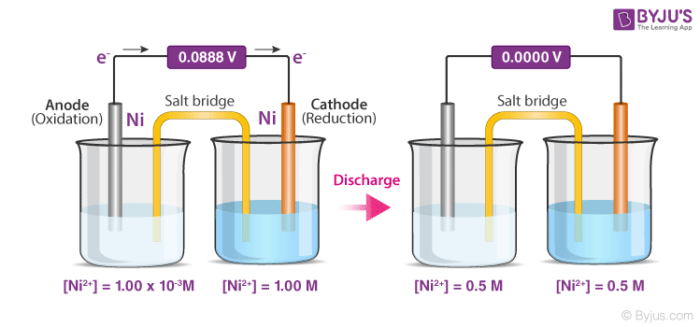
Concentration cells can be defined as electrochemical cells that consist of two half-cells wherein the electrodes are the same, but they vary in concentration. As the cell as a whole strives to reach equilibrium, the more concentrated half cell is diluted and the half cell of lower concentration has its concentration increased via the transfer of electrons between these two half cells. Therefore, as the cell moves towards chemical equilibrium, a potential difference is created. A detailed diagram of a concentration cell and its discharge process is given below.
Types of Concentration Cells
Concentration cells can be classified into two types, namely:
- Electrode Concentration Cells
- Electrolyte Concentration Cells
Electrode Concentration Cells
These cells consist of identical solutions used as electrolytes in each half-cell. However, the half-cells differ in the concentration of the electrode (the electrodes are made up of the same material).
An Example for this type of cell would be a cell consisting of two hydrogen electrodes which are subjected to varying pressures but are immersed in the same solutions (containing hydrogen ions).
Electrolyte Concentration Cells
These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. In these cells, the electrolyte tends to diffuse from higher concentration solutions towards solutions of lower concentration.
An example for this type of cell is a cell where the anode consists of Zn/Zn2+(0.1M) whereas the cathode consists of Zn2+(0.01M)/Zn. In this cell, the flow of electrons from the anode to the cathode is due to the reduction of Zn2+ ions at the cathode into metallic zinc.
Components of the Concentration Cell
Salt Bridge
The salt bridge offers the perfect solution for the separation of the two half-cells while providing a pathway for ion transfer. Electric wires would react with the ions flowing through them. The absence of a salt bridge would also lead to a build up of electrons in one half cell from the incoming flow of electrons belonging to the other half cell.
Electrode
The two electrodes are called the cathode (right side) and the anode (left side). The anode loses electrons and is the site where the oxidation occurs, whereas the cathode is the area where the electrons accumulate and the reduction occurs.
Voltmeter
The voltmeter is used to measure the cell potential of the cell. Cell potential is also referred to as electromotive force (or EMF). The voltmeter is generally placed in-between the two half-cells.
To conclude, the concentration cell is a type of galvanic cell where the half-cells consist of the same substance but at different concentrations. These cells give a small potential difference while moving towards chemical equilibrium which can be measured using a voltmeter.

Comments