What are conductors and insulators?
Solids can be classified as conductors, insulators, and semiconductors. The flow of charge is defined as the electric current, and those materials that readily allow the flow of charge through them are termed as conductors. The materials that do not permit the flow of current through them are referred to as insulators. So the ability to allow current to pass through them is termed as the conductivity of the material. It typically ranges from 10-20 to 107 ohm-1m-1 for solids.
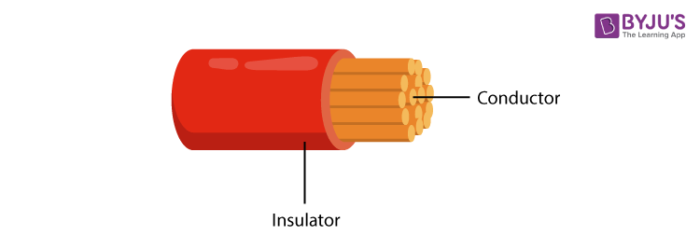
Those solids which have conductivity ranging from 104 to 107 ohm-1m-1 are considered as conductors. Most of the metals which have the conductivity of 107 ohm-1m-1 are regarded as good conductors of electricity.
Different materials have the different type of arrangement of the atoms, and each atom has its degree of freedom. In metals, the outermost electron in an atom is loosely packed and therefore can move freely in the material. Because of these free electrons, metals conduct electricity and are called as conductors. On the other hand, insulators are those materials in which the electrons are tightly bound and are not free to move, e.g. glass.
In the solid state, the electrical conductivity of solids is explained by the band theory. There are two bands in a solid, conduction band and valence band. These two bands are close to the Fermi level. The valence band contains highest energy electrons and in this group, there is the presence of electrons even at absolute zero temperature. The band which has the lowest range of vacant electronic state is called as the conduction band. The electronic band structure is such that the valence band is below the Fermi level, and the conduction band is above the Fermi level.
In conductors, the valence band and the conduction band overlap each other, and the gap between them is negligible because of which there is the maximum transfer of electrons, and thus they have excellent conductivity. In insulators, there is a large difference between these two bands which results in no transfer of electrons and that’s why insulators are a bad conductor of electricity.
Recommended Videos
Solid State

For more information on this topic log on to BYJU’S.

Comments