What is Dehydration of Alcohol?
Hydroxy derivatives of hydrocarbons in which one or more hydrogen atoms are replaced by an equal number of hydroxyl groups are called Alcohols.
Alcohol upon reaction with protic acids tends to lose a molecule of water to form alkenes. These reactions are known as dehydrogenation or dehydration of alcohols.
It is an example of an elimination reaction. Its rate varies for primary, secondary and tertiary alcohols. This variation of rate can be attributed to the stability of carbocation generated. Since the carbocation is most stable in the case of tertiary alcohols, the rate of dehydration is highest for tertiary alcohols in comparison to secondary and primary alcohols.
Dehydrogenation is the removal of hydrogen from the feedstock, such as the treatment of paraffin for the production of olefin. The degree of dehydrogenation during thermal cracking of petroleum varies with the starting material and operating conditions, but due to its practical significance, methods have been found to increase the level of dehydrogenation and, in some cases, to make it almost the only reaction.
Table of Contents
Dehydrogenation
Dehydrogenation is one of the most important processes in the chemistry of petroleum because it turns the starting inert alkanes into olefins and aromatic compounds, starting points towards other functional groups.
Catalytic dehydrogenation plays a significant role in the development of olefin light (C3–C4 carbon range), detergent range (C10–C13 carbon range), and dehydrogenation to styrene by ethylbenzene. During the Second World War, catalytic dehydrogenation of butane over a catalyst for chromium – alumina was practised to generate butenes that were dimerized to octenes and hydrogenated to octans to yield high octane aviation fuels.
Dehydrogenation is a highly endothermic process, and as such, a restricted reaction to the equilibrium. Important aspects of dehydrogenation include reaching equilibrium or conversion to near-equilibrium while reducing side reactions and coke formation.
Recommended Videos

Dehydrogenation Reaction
Dehydration Mechanism Steps
If either Raney-Ni, Al(i-ORr)3, or alumina are absent from the catalytic mixture, secondary alcohol dehydrogenation reaction to ketones does not occur. When the Raney-Ni is substituted with other Ni(II) salts I e. NiCl2) or complexes I e. Ni(PPh3)2Cl2), no reaction is noted.
Dehydration of alcohols follows a three-step mechanism.
-
-
-
- Formation of protonated alcohol
- Formation of carbocation
- Formation of alkenes
-
-
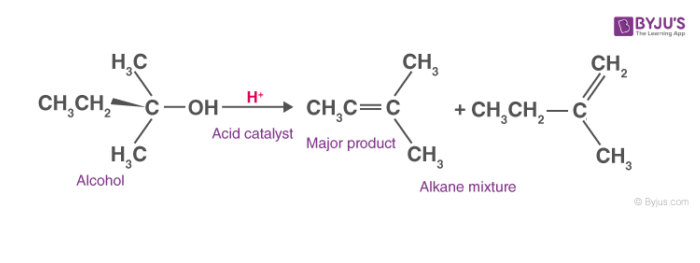
General dehydration reaction of alcohols can be seen as,
Mechanism of Dehydration of Alcohols:
Dehydration of alcohols can follow E1 or E2 mechanisms. For primary alcohols, the elimination reaction follows E2 mechanism while for secondary and tertiary alcohol elimination reaction follows E1 mechanism.
Generally, it follows a three-step mechanism. The steps involved are explained below.
1. Formation of protonated alcohol:
In this step, the alcohol is acted upon by a protic acid. Due to the lone pairs present on the oxygen atom it acts as a Lewis base. Protonation of alcoholic oxygen takes place which makes it a better leaving group. It is a reversible step which takes place very quickly.

2. Carbocation formation:
In this step, the C-O bond breaks generating a carbocation. This step is the slowest step in the mechanism of dehydration of an alcohol. Hence, the formation of the carbocation is considered as the rate-determining step.
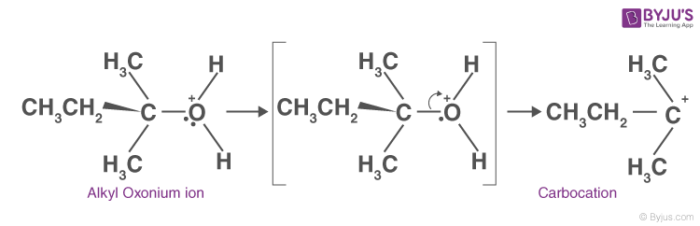
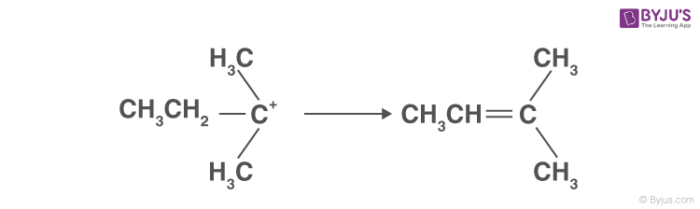
3. Alkene formation:
This is the last step in the dehydration of alcohol. Here the proton generated is eliminated with the help of a base. The carbon atom adjacent to the carbocation breaks the existing C-H bond to form C=C. Thus, an alkene is formed.
Dehydrogenation and rehydrogenation reactions are reversible and the reagents and components are recyclable as a result of which the device can be used more efficiently as a hydrogen supply network technology compared to other hydrogen storage materials.
Frequently Asked Questions – FAQs
How are alkenes prepared?
Alkenes are typically prepared by means of β elimination reactions, in which two atoms are removed on neighbouring carbon atoms, resulting in a double bond formation. Preparations include alcohol oxidation, alkyl halides dehydrohalogenation and alkane alkaline dehalogenation.
How do you convert alkanes to alkenes?
An alkene is an unsaturated hydrocarbon with double bonds, while an alkane is a saturated hydrocarbon with single bonds only. To transform an alkane to an alkene, at extremely high temperatures, you need to extract hydrogen from the alkane molecule. The cycle is called dehydrogenation.
What is the process of hydrogenation?
Hydrogenation is the mechanism where, in the presence of a catalyst, the hydrogen atoms bind to a compound’s double bond, allowing its conversion to a single bond. Hydrogenation is commonly used during food products manufacturing where unsaturated fats and oil are converted into saturated fats and oils.
What is hydrogenation and its application?
Hydrogenation in the presence of a catalyst is the chemical reaction between the hydrogen and other compounds. For example; hydrogenation is used in Petrochemical Industry to turn alkenes into alkanes (paraffins) and cycloalkanes. The hydrogenation of vegetable ghee from vegetable oils is often used to produce.
How does dehydrogenation relate to oxidation?
As the detached hydrogen is immediately oxidized (oxidative dehydrogenation), the conversion of the reactants to the products is increased as the concentration of the equilibrium is transferred towards the products and the added exothermic oxidation reaction provides the required heat for the reaction.
What is the difference between dehydration and dehydrogenation?
Dehydration is a reaction involving the removal or release of one or more molecules of water while dehydrogenation is a reaction involving the removal of one or more molecules of hydrogen.
How do you compare the rate of dehydration?
The rate of dehydration is related to the ease of carbohydrate formation and the energy of the intermediate carbohydrate. The ease of carbohydrate formation is tertiary > secondary > primary.
For a detailed discussion on dehydration of alcohols, please download BYJU’S – The Learning App.

Comments