The diazotization reaction mechanism generally involves the usage of nitrous acid and another acid in the treatment of aromatic amines in order to yield the diazonium salt.
What is Diazotization?
The chemical process used in converting a primary aromatic amine into the corresponding diazonium salt of the amine is commonly referred to as diazotization.
The German industrial chemist Peter Griess was the first person to report such a reaction in 1858. He went on to discover many more reactions involving diazonium salts.
Generally, the preparation of these diazonium salts involves the reaction of an aromatic amine with nitrous acid in the presence of another acid. An example of a diazotization reaction is given below.
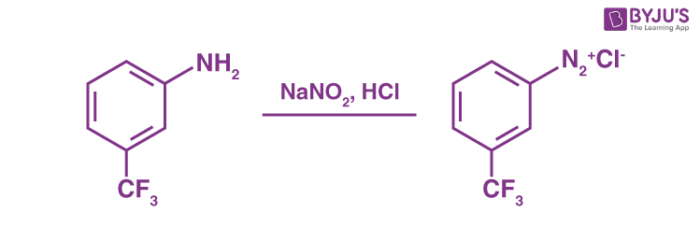
In the example illustrated above, it can be noted that nitrous acid is generated from the reaction between the sodium nitrite and the other mineral acid (acid derived from one or many inorganic compounds) which is present in excess.
Diazotization Reaction Mechanism
The diazotization reaction mechanism begins with the reaction of nitrous acid with the other acid to give water and nitrosonium ion.
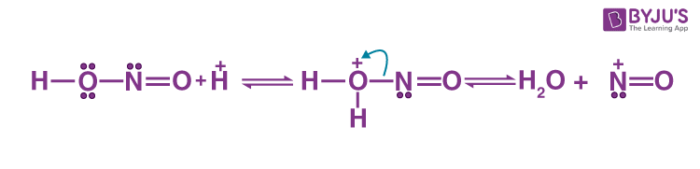
Thus, the required nitrosonium ion is formed. This is now reacted with the aromatic ring to which the NH2 group is attached. The positive charge of the nitrosonium ion is now shifted to the nitrogen which is directly attached to the aromatic ring as a new nitrogen-nitrogen bond is formed. The subsequent deprotonation gives the n-nitrosamine.
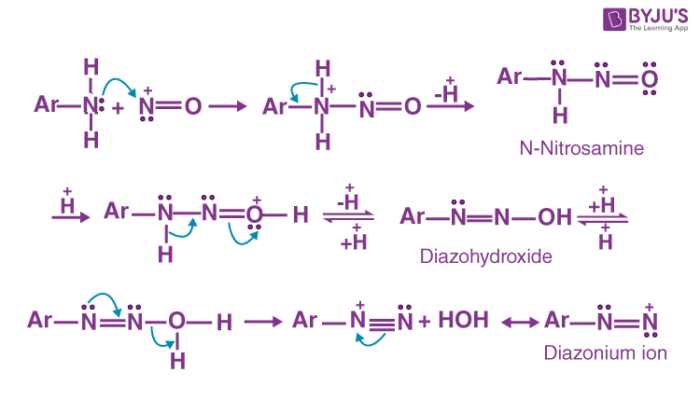
In the presence of excess acid, n-nitrosamine can be converted into a diazohydroxide by protonation and subsequent deprotonation. Diazohydroxide is now protonated and water is removed from the compound to give the required aryl diazonium ion (which can easily be converted into a diazonium salt).
The mechanism of the diazotization reaction of aniline is illustrated below.
Uses of Diazonium Compounds
A few applications of diazonium compounds have been listed below.
- Diazonium compounds are very useful in the dye and pigment industries. They were initially used in the production of water-fast dyed fabrics.
- Diazonium Salts are found to be useful in the Fischer Indole Synthesis process because they can be reduced to hydrazine derivatives with the help of stannous chloride.
- They are used as standard reagents while synthesizing organic compounds.
- Diazonium salts have the potential to have applications in the field of nanotechnology. They are useful in the efficient functionalization of single-wall nanotubes.
Frequently Asked Questions
Define diazotization.
Which reagent is used for diazotization?
Give two uses of diazotization compounds.
They are used as standard reagents while synthesizing organic compounds
Name the ion formed during the first step of the diazotization reaction mechanism.
What product is formed during the diazotization reaction?
Thus, the diazotization reaction mechanism is very important in organic chemistry. Click here to learn more about the Chemical Reactions of Diazonium Salts and their properties.

Comments