What is Chromatography?
Chromatography is the technique for the separation, purification, and testing of compounds.
The term “chromatography” is derived from Greek, chroma meaning, “colour,” and graphein meaning “to write.”
In this process, we apply the mixture to be separated on a stationary phase (solid or liquid) and a pure solvent such as water or any gas is allowed to move slowly over the stationary phase, carrying the components separately as per their solubility in the pure solvent.

Table of Contents
- Principles of Chromatography
- Types of Chromatography
- Related Topics on Chromatography
- What is Differential Extraction?
- Applications of Chromatography
- Frequently Asked Questions
Principles of Chromatography
Chromatography is a separation method where the analyte is combined within a liquid or gaseous mobile phase., which is pumped through a stationary phase. Usually one phase is hydrophilic and the other is lipophilic. The components of the analyte interact differently with these two phases. Depending on their polarity they spend more or less time interacting with the stationary phase and are thus retarded to a greater or lesser extent. This leads to the separation of the different components present in the sample. Each sample component elutes from the stationary phase at a specific time called as retention time. As the components pass through the detector their signal is recorded and plotted in the form of a chromatogram.
Types of Chromatography
The four main types of chromatography are
1. Adsorption Chromatography
In the process of adsorption chromatography, different compounds are adsorbed on the adsorbent to different degrees based on the absorptivity of the component. Here also, a mobile phase is made to move over a stationary phase, thus carrying the components with higher absorptivity to a lower distance than that with lower absorptivity. The main types of chromatographic techniques that are used in industries are given as under.

2. Thin Layer Chromatography
In the process of thin-layer chromatography (TLC), the mixture of substances is separated into its components with the help of a glass plate coated with a very thin layer of adsorbent, such as silica gel and alumina, as shown in the figure below.
The plate used for this process is known as chrome plate. The solution of the mixture to be separated is applied as a small spot at a distance of 2 cm above one end of the plate. The plate is then placed in a closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.

3. Column Chromatography
Column chromatography is the technique used to separate the components of a mixture using a column of suitable adsorbent packed in a glass tube, as shown in the figure below. The mixture is placed on the top of the column, and an appropriate eluant is made to flow down the column slowly.
Depending upon the degree of adsorption of the components on the wall adsorbent column, the separation of the components takes place. The component with the highest absorptivity is retained at the top, while the other flow down to different heights accordingly.

Column Chromatography
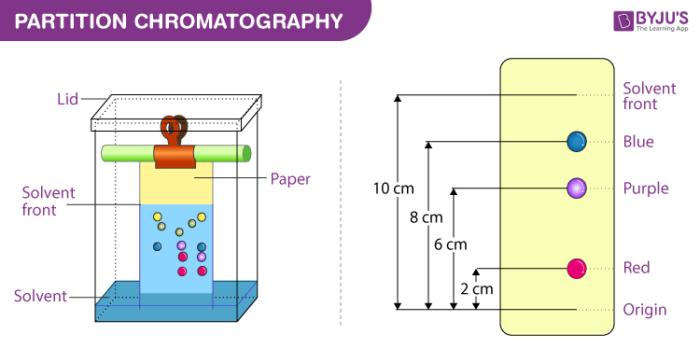
4. Partition chromatography
In this process, a continuous differential partitioning of components of a mixture into a stationary phase and mobile phase takes place. The example of partition chromatography can be seen in paper chromatography. In this process, chromatography paper is used as a stationary phase which is suspended in a mixture of solvents that act as a mobile phase.
Here, we put a spot at the base of the chromatographic paper with the mixture to be separated and as the solvent rises up this paper, the components are carried to different degrees depending upon their retention on the paper. The components are thus separated at different heights.
Related Topics on Chromatography
- Adsorption Chromatography
- Thin Layer Chromatography
- Column Chromatography
- Partition Chromatography
- Applications of Chromatography
What is Differential Extraction?
Differential extraction is the method of separation of any organic component present in an aqueous solution. In this process, we use an organic solvent for which the solubility of the desired compound is more than compared to that in water. Also, the organic solvent is chosen such that it is immiscible with the aqueous solution so that it can form layers and can be separated easily using a separating funnel.
The organic compound is later recovered by the process of distillation or evaporation. The process of continuous extraction is used in cases when the solubility of the compound is less in the organic solvent.

Applications of Chromatography
In bio analytical chemistry, chromatography is mainly used for the separation, isolation and purification of proteins from complex sample matrices. In cells for example, proteins occur alongside numerous other compounds such as lipids and nucleic acids. In order to be analysed, these proteins must be separated from all the other cell components. Then the proteins of interest might have to be isolated from other proteins and purified further.
Chromatography is an essential part of almost any protein purification strategy. A number of different chromatographic techniques are used for the purification and analysis of proteins. They can be classified according to the physical principle involved in the separation process. Typical examples include reversed phase chromatography, ion exchange chromatography, affinity chromatography and size exclusion chromatography.

Frequently Asked Questions – FAQs
What is the basic principle of chromatography?
Chromatography is based on the concept of separating molecules in a mixture added to the ground or solid and liquid stationary state (stable phase) when travelling with the aid of a mobile phase.
What is the Rf value in chromatography?
RF stands for retention factor in paper chromatography, or the distance a fluid compound moves up a plate of chromatography. For each particular solvent, all compounds have a common RF value, and RF values are used to equate unknown samples with known compounds.
How is RF value useful?
The distance travelled by sample divided by the distance travelled by the solvent. It is a component characteristic and can be used to classify components for a given system at a known temperature.
Where is chromatography used?
Chromatography is used in industrial processes to purify materials, test trace amounts of contaminants, isolate chiral compounds and quality control test products. Chromatography is the physical process of separating or analysing complex mixtures.
To learn more about differential extraction and chromatography and other separation techniques, download BYJU’S The Learning App.


It was wonderful time I spent
Excellent message
It’s interesting to know that chromatography is used in an industrial process to purify materials and test trace amounts of contaminants to control the quality of the products. My father is looking for a portable HLPC system for his work, and I was curious about what it was for, which is why I’ve been researching about it. That’s why I really appreciate this article because I learned a lot about chromatography. Thanks!
VERY USEFUL TOPIC IN PHARMA AND OTHER INDUSTRY