What is Electrochemistry?
Electrochemistry can simply be defined as the study of chemical reactions that cause electrons to move about. The movement of electrons from one element to another is called a “Redox Reaction”. In a redox reaction, there is a change in the oxidation state of one or more elements. The simplest example of an electrochemical reaction is the AA batteries that we use to power up our daily appliances.
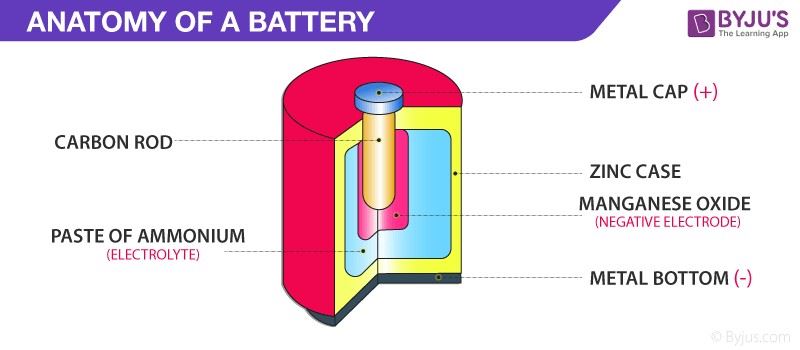
The above image shows the basic layout of an electrochemical battery, which you can read more about in our electrochemical cell PDF. From the simplest reactions such as batteries to the nerve cells strewn across all of our body, we know that we are surrounded by electrochemistry wherever we go. For more information on the wondrous properties of electrochemistry, check out our electrochemistry PDF below.
Recommended Videos
Introduction To ElectroChemistry – Galvanic Cell

Electrochemistry – One Shot & Mind Maps

Conductors, Conductance & Conductivity
Galvanic Cell, Electrolytic Cell, EMF of Cell & Faraday’s Laws
ElectroChemistry Class 12 Chemistry – Important Topics for JEE Main

To learn more about important concepts related to electrochemistry, such as the Nernst equation, download BYJU’S – The Learning App.

Comments