Group 16 elements in the modern periodic table are known as the oxygen family. The first four elements of this group are nonmetals, and they are termed as ‘the chalcogens’ or they can also be referred as ore-forming elements as most of the metal ores are oxides or sulfides. This group consists of oxygen (O), sulfur (S), selenium (Se), tellurium (Te) and polonium (Po) (a radioactive element). These elements exist in free as well as in combined states.
In the electronic configuration, the word ‘electronic’ means electron and ‘configuration’ means an arrangement of something. It depicts the arrangement of electrons in the orbital shells and subshells. Before starting with filling the electron diagram we should know some basic rules for the same. It is compulsory to fill the lowest energy level first and then we need to proceed towards the higher energy levels. Pauli’s exclusion principle, Hund’s rule, and Aufbau’s principle should be considered during the arrangement of electrons. Pauli’s exclusion principle states that any two electrons in an atom cannot have the same four quantum numbers (n, l, m, s). The starting three can be the same but the fourth one (s) can never be the same. According to Hund’s rule, when electrons are arranged in orbitals, then similar energy orbitals first occupy one electron in each of them, and then only the electrons can start pairing with other electrons in a half-filled orbital. Lastly, according to Aufbau’s principle, the electron will occupy the lowest energy level first. The electronic configuration of chemical elements in the modern periodic table can be written by considering the above principle and rules.
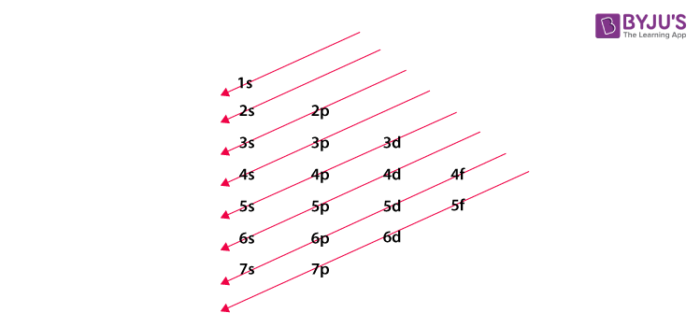
Atomic orbital diagonal rule
Now that we know all the principles and rules, we can write the electronic configuration of an element. As we are reading about chalcogen elements, so we will start with oxygen. The atomic number of oxygen is 8. In this case, first, we will fill the 1s orbital with two electrons (as it is the lowest energy level), and then we will fill the 2s and 2p orbital with 2 and 4 electrons respectively. So its electronic configuration can be written as 1s22s22p4. Neil Bohr said that the electronic configurations of the elements of the same group are similar to each other. So by noting this point, we can give the configuration of other elements as shown in the table:
Atomic number |
Element |
Configuration |
| 8 | O | [He]2s2 2p4 |
| 16 | S | [Ne]3s2 3p4 |
| 34 | Se | [Ar]3d10 4s2 4p4 |
| 52 | Te | [Kr]4d10 5s2 5p4 |
| 84 | Po | [Xe]4f14 5d10 6s2 6p4 |
Looking at the table we can say that the number of valence electrons of elements of chalcogen elements is 6. The symbol of elements written in the square bracket is the nearest noble gas element. Now, we can say that the general electronic configuration of group 16 elements is ns2np4.
Click here, to know more about group 16 elements of the periodic table.


Comments