Alkenes belong to the group of unsaturated hydrocarbons that is one molecule of alkene contains at least one double bond. Due to the presence of pi electrons they show addition reactions in which an electrophile attacks the carbon-carbon double bond to form the addition products. These reactions are known as electrophilic addition reactions of alkenes. Sometimes these addition reactions follow free radical mechanism too. Oxidation and ozonolysis reactions are also some prominent reactions of alkenes. Some of these reactions are discussed below:
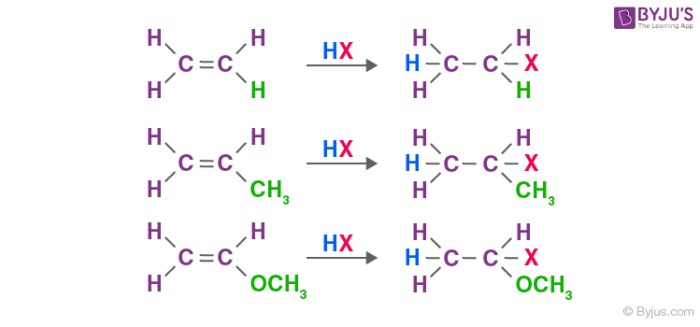
Electrophilic addition reactions of alkenes: Alkenes exhibit wide range of electrophilic addition reactions. Addition of hydrogen halides such as hydrogen bromide and hydrogen chloride is an example of electrophilic addition reactions of alkenes. The general trend of hydrogen halide is given as: HI >HBr> HCl. For symmetrical alkenes such as ethene it is quite easier to predict the end product in comparison to unsymmetrical alkenes such as propene. For example:
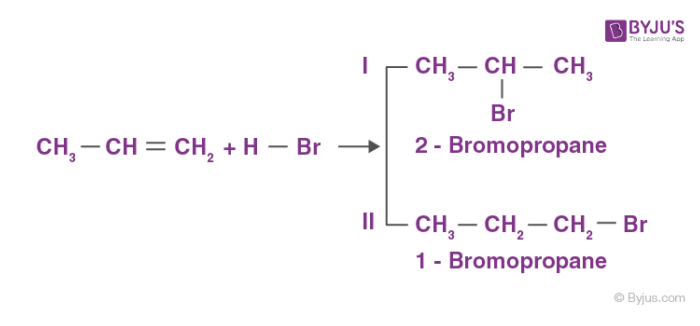
Markovnikov proposed a rule called the Markovnikov rule for the prediction of major products in such cases. Markovnikov rule states that the negative part of the adding molecule gets attached to that carbon atom which possesses a lesser number of hydrogen atoms. Thus, 2-bromopropane is the expected product by this rule. This can further be explained with the help of the mechanism of electrophilic substitution reactions of alkenes. The general mechanism is explained below:
An electrophile, H+, is generated from hydrogen bromide, which attacks the double bond to form a carbocation.
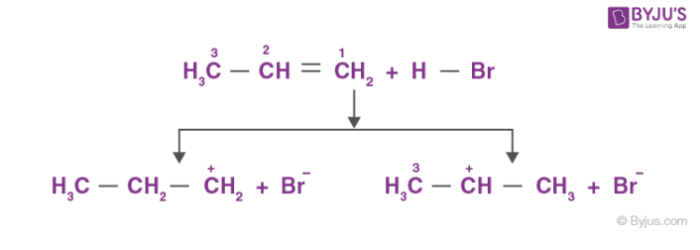
Since secondary carbocation is more stable than primary carbocation, secondary carbocation predominates the formation of ions.
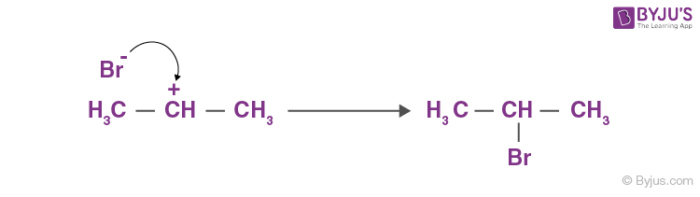
Finally, Br– attacks the carbocation to form alkyl halides.
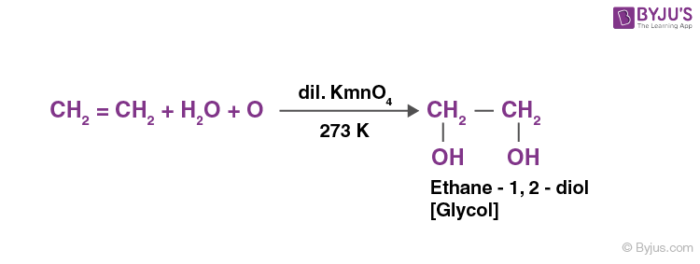
The oxidation reaction of alkenes: Oxidation of alkenes leads to the formation of ketones and alcohols. For example in the presence of a cool, aqueous solution of potassium permanganate, alkenes oxidize to vicinal glycols.
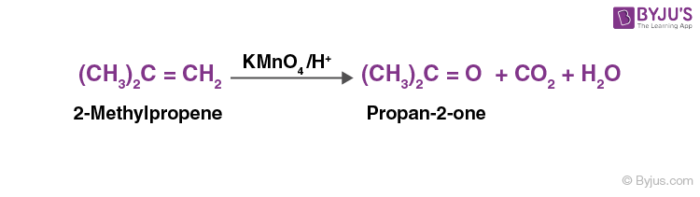
In the presence of acidic potassium permanganate, alkenes oxidize to ketones or acids.
Frequently Asked Questions – FAQs
Is hydrogenation electrophilic addition?
There is another alkene reaction, hydrogenation, which merits notice but is not related to the mechanism of electrophilic addition. The addition of molecular hydrogen (H2) to the alkene double bond is termed hydrogenation. It transforms a simple alkene into an alkane.
What is the difference between electrophilic addition and nucleophilic addition?
Electrophilic addition is where an electron pair is embraced by the group being introduced, while nucleophilic addition is where an electron pair is donated by the added group.
Why do alkenes undergo electrophilic addition reactions?
Alkenes are doubly bound and sp2 hybridized, which can be donated to an electrophile, such as electrophilic addition, by the electrons in the side-to-side overlap of p orbitals that allows the pi bond.
Why can’t alkanes undergo an addition reaction?
Alkanes are not undergoing addition reactions, and they only have single σ bonds now, so they do not get more structurally stable or stronger-they are already at their height, and so they can only switch things about in replacement reactions.
What is meant by an addition reaction?
In organic chemistry, an external reaction is an organic reaction in the simplest terms, where two or more molecules interact to form a stronger one (the adduct). Two major forms of polar addition reactions are available: electrophilic and nucleophilic addition.
For detailed discussion on nomenclature of alkanes, alkenes and alkynes, download BYJU’S – the learning app.’

Comments