Resonance involved in the benzene ring makes the delocalized electron span effectively over the carbon atoms in the benzene ring. It partially stabilizes the arenium ion too. Partial stability of arenium ion makes benzene highly prone to electrophilic substitution reactions.
Table of Contents
What is Electrophilic Substitution of Benzene?
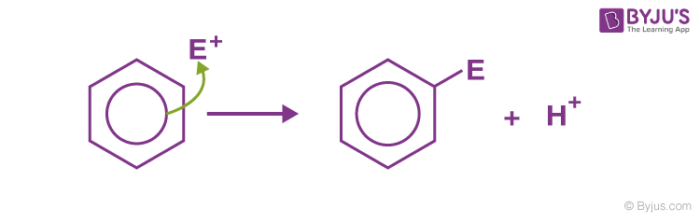
Electrophilic substitution of benzene is the one where an electrophile substitutes the hydrogen atom of benzene. As the aromaticity of benzene is not disturbed in the reaction, these reactions are highly spontaneous in nature. Basic examples of electrophilic substitution reaction of benzene are nitration, sulfonation, halogenation, Friedel Craft’s alkylation and acylation, etc.
Recommended Videos
The Mechanism for Electrophilic Substitution of Benzene
An electrophilic substitution reaction generally involves three steps:
1. Generation of electrophile: In the presence of Lewis acid, generation of electrophile takes place. As the Lewis acid accepts the electron pair from the attacking reagent.
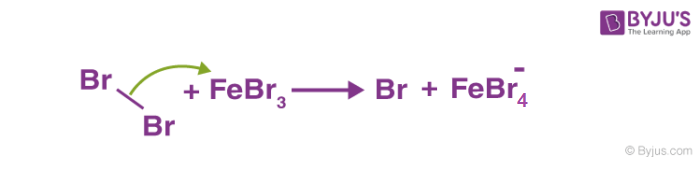
2. Formation of arenium ion: The electrophile generated attacks on the benzene ring to form a positively charged cyclohexadienyl cation better called an arenium ion containing one sp3 hybridized carbon atom. The positive charge is effectively distributed over three carbon atoms by resonance which makes it partially stable.
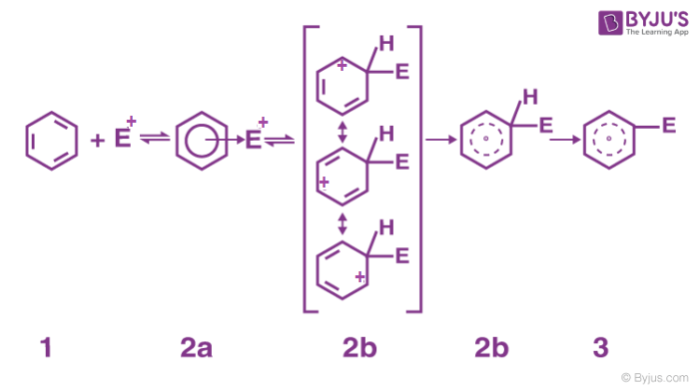
As the delocalization of electrons stops at an sp3 hybridized carbon atom, the arenium ion is not aromatic in nature.
3. Removal of positive charge from the carbocation intermediate: The arenium ion finally loses its proton from sp3 hybridized carbon to a Lewis base restoring the aromaticity.
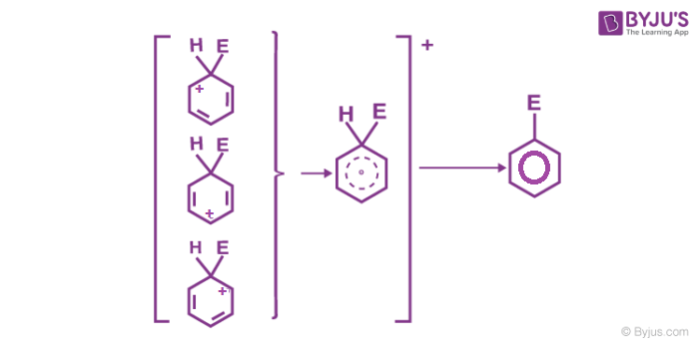
Few examples of electrophilic aromatic substitution
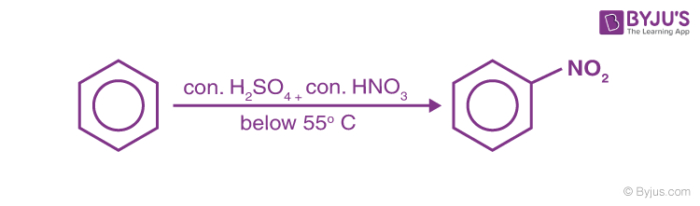
1. Nitration of Benzene
Benzene reacts with nitric acid at 323-333k in presence of sulphuric acid to form nitrobenzene. This reaction is known as nitration of Benzene.
2. Sulphonation of Benzene
Sulphonation of benzene is a process of heating benzene with fuming sulphuric acid (H2SO4 +SO3) to produce benzenesulphonic acid. The reaction is reversible in nature.

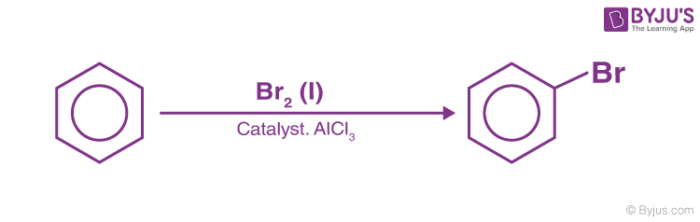
3. Halogenation of Benzene
Benzene reacts with halogens in the presence of Lewis acid like FeCl3, FeBr3 to form aryl halides. This reaction is termed halogenation of benzene.
Frequently Asked Questions – FAQs
Why does benzene undergo electrophilic substitution?
What are the three steps involved in the electrophilic substitution of benzene?
Step 2: Formation of the carbocation.
Step 3: Removal of the proton.
What is an electrophilic substitution reaction? Give example
Examples of such reactions include aromatic nitrations, aromatic sulphonation, and Friedel-Crafts reactions
What are the products of nitration, chlorination and Friedel Craft alkylation of benzene ?
Join BYJU’S to witness the most innovative ways of learning.

Comments