Introduction
The energy of orbitals refers to the energy required to take an electron present in that orbital to infinity or the energy released when an electron is added to that orbital from infinity. The energy of orbital depends on principle quantum number (n) and azimuthal quantum number (l) i.e. it depends on shell and subshell.
Table of Contents
- Recommended Videos
- Energy of Orbital in Hydrogen (single-electron atom)
- Energy of Orbital in Multi-Electron Atom
Recommended Videos

For orbitals belonging to the same subshell, it is same and those orbitals with the same energy are known as degenerate orbitals.
Energy of Orbital in Hydrogen (single-electron atom)
- The exception to the general behaviour of the energy of orbitals as explained above is observed in Hydrogen, the energy of orbital is only dependent on principal quantum number, and so the 2s and 2p orbital in hydrogen atom have the same energy.
- The 1s orbital in hydrogen atom corresponds to the most stable condition and is called ground state whereas any other orbital afterwards has higher energy than that of 1s orbital and are called excited state.
Energy of Orbital in Multi-Electron Atom
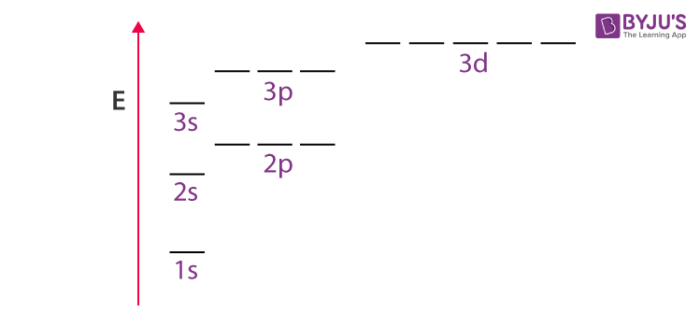
Unlike hydrogen (single-electron atom), multi-electron atoms tend to have a different energy in different subshells of the same shell. The energy of orbital in these types of atoms is dependent on both principal quantum number (n) or shells and azimuthal quantum number (l) or subshells. That is, for a given principal quantum number let’s say 3, the different subshells 3s, 3p and 3d will have different energies.
The reason behind different energies between the various subshells of the same shell is that there exists a mutual repulsion among the electrons in multi-electron atoms. The stability of multi-electron atom is due to the bigger magnitude of attractive force between nucleus and electrons as compared to the forces of repulsion between electrons of the inner shell and outer shell.
Due to the presence of electrons in the inner shell, the outer shell electrons are not able to experience a full positive charge of the nucleus. This effect is known as the shielding effect and the net nuclear charge felt by an outer shell electron is known as an effective nuclear charge.
For more information on the energy of orbitals, shielding effect and effective nuclear charge, download BYJU’S – The learning app to play store and app store.

Comments