What are Epimers?
Epimer in stereochemistry specifies one of a pair of stereoisomers. At the stereogenic centre, two isomers present in the molecule differ, while the rest remains identical. A molecule may contain numerous stereocenters leading to several stereoisomers.
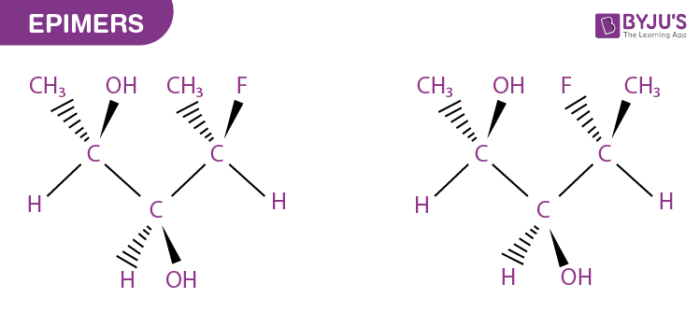
Stereoisomers are isomeric molecules that possess the same constitution and molecular formula, but they vary in three-dimensional orientations of their atoms in space.
Epimers – Example
Below, stereoisomers illustrate the D and L configurations of glucose. Here, glucose is referred the D and L on the basis of last chiral carbon atom.
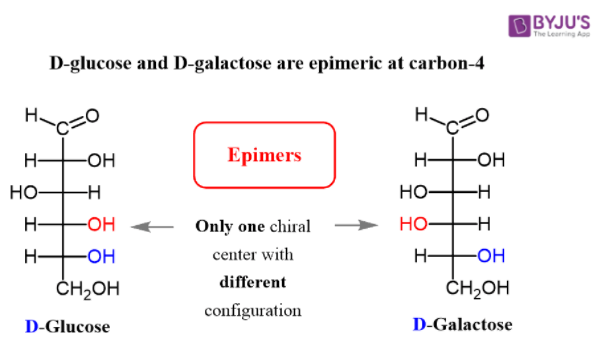
Glucose involves the formation of glycogen, starch, glucose, oligosaccharides and polysaccharides. Due to the presence of carbon in glucose molecule it may exhibit stereoisomerism, that is enantiomers and diastereomers.
Enantiomers
Optical isomers or enantiomers are two isomers that are relevant to each other by reflection. They are non – superimposable mirror images of each other. They are comprised of the same physical properties except in a way they interact with several optical isomers of other compounds. Hence, different optical isomers may have variant biological effects.
Diastereomers
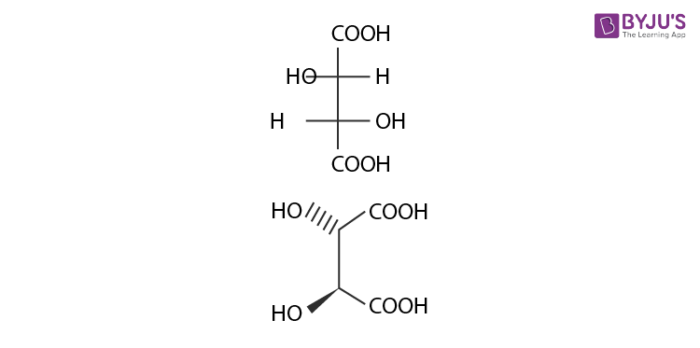
Diastereomers are stereoisomers that are not mirror images of each other in that they are not linked with reflection operation unlike of enantiomers. They possess the same physical properties. One of the example include meso compounds. The below structure is mesotartaric acid.
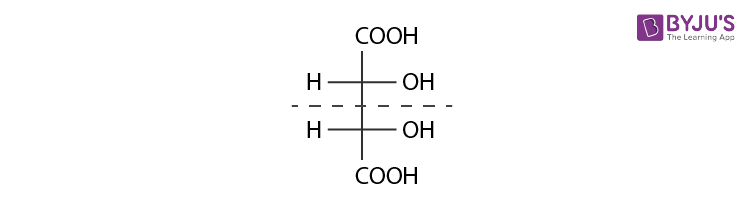
Below example illustrates, formation of enantiomeric pair, where mesotartaric acid forms diastereomeric pair with dextro tartaric acids and levo.
- Dextrotartaric acid
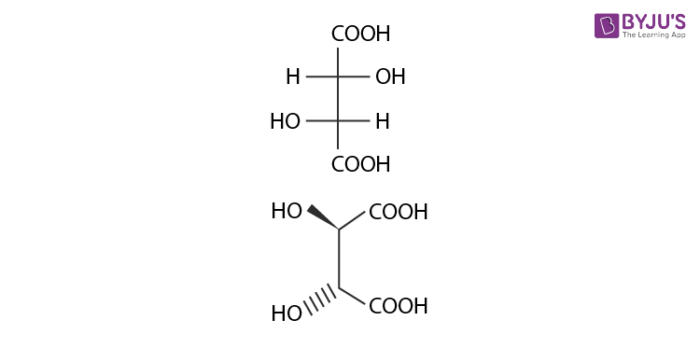
- Levotartaric Acid
Epirubicin and Doxorubicin are epimers that are used in drugs.
Frequently Asked Questions – FAQs
What are Epimers with examples?
Epimers are carbohydrates that differ in the location of the -OH group in one location. Both D-glucose and D-galactose are the best examples. D-glucose and D-galactose epimers create a single difference at C-4 carbon. They are not enantiomers, they are just epimers, or diastereomers, or isomers.
What are Epimers in biochemistry?
One of a pair of stereoisomers that differ from a single stereocenter’s absolute configuration. If there is only one stereocenter in the molecule, so the epimers are enantiomers. If there are two or more stereocenters in the molecule, so the epimers are diastereomers.
Which is the simplest carbohydrate?
Monosaccharides, or basic sugars, are considered the simplest carbohydrates. Glucose is an example. To make bigger molecules, monosaccharides may be united. Two monosaccharides unite to form one disaccharide.
What is the difference between epimers and enantiomers?
At one of the chiral centres inside the molecules, epimers have opposite stereochemical arrangements. Enantiomers at all the chiral centres inside the molecules have opposite stereochemical configurations. Epimers, for instance, are D-glucose and D-galactose.
What is Mutarotation explain with example?
Mutarotation, as the corresponding stereocenters interconvert, is the change in optical rotation due to the change in equilibrium between two anomers. As x-alpha and β anomeric forms interconvert, cyclic sugars exhibit mutarotation.

Comments