What is Hinsberg Reagent?
Hinsberg reagent is an alternative name for benzene sulfonyl chloride. This name is given for its use in the Hinsberg test for the detection and distinction of primary, secondary, and tertiary amines in a given sample.
This reagent is an organosulfur compound. Its chemical formula can be written as C6H5SO2Cl. The appearance of the Hinsberg reagent can be described as a colourless oil that is viscous in nature and is soluble in organic solvents.
This Reagent undergoes a reaction with compounds that contain O-H and N-H bonds that are reactive in nature. It is used in the preparation of sulfonamides (via reaction with amines) and sulfonamide esters (via reaction with alcohol).
Recommended Video
Identification, Nomenclature and Classification of Amines – Primary, Secondary and Tertiary Amines

Preparation of Hinsberg Reagent
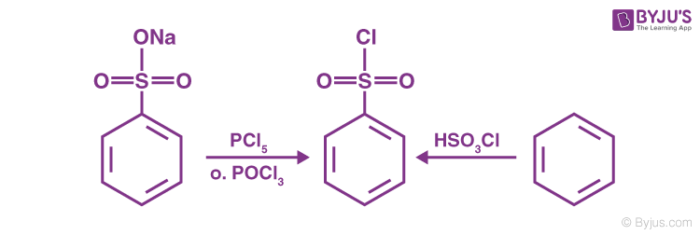
The chlorination of benzene sulfonic acid or the salts of benzene sulfonic acid with phosphorus oxychloride (POCl3) gives the required reagent.
Another way to prepare the required Hinsberg Reagent is by reacting benzene with chloro sulfuric acid (chemical formula HSO3Cl). Both these methods for the preparation of the required reagent are illustrated below.
Hinsberg Test
Hinsberg test is a chemical reaction used to distinguish between primary, secondary, and tertiary amines. This reaction was described first in 1890 by the German chemist Oscar Heinrich Daniel Hinsberg.
In the Hinsberg Test, the amines act as nucleophiles and attack the electrophile (sulfonyl chloride). This leads to the displacement of the chloride and the generation of the sulfonamides. When primary and secondary amines form sulfonamides, this sulfonamide product is not soluble and precipitates from the solution as a solid.
Hinsberg Reaction Pathways
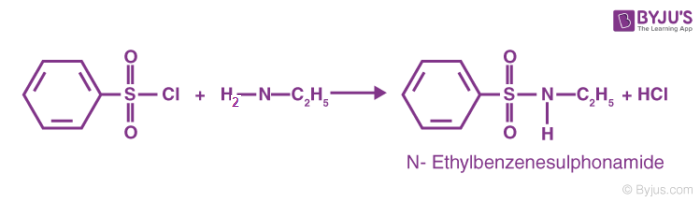
The reaction of the benzene sulfonyl chloride with primary amines gives a sulfonamide product that is soluble in alkali. This reaction can be illustrated as follows.
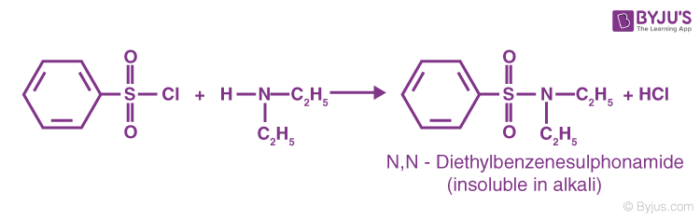
The reaction of the benzene sulfonyl chloride with secondary amines gives a sulfonamide product that is NOT soluble in alkali. An example of this type of reaction is given below.
No such reaction occurs between a tertiary amine and the benzene sulfonyl chloride reagent. Tertiary amines help in the hydrolysis of sulfonyl chloride. This reaction yields salts that are soluble in water.
Thus, the Hinsberg reagent can be used to react with primary, secondary, and tertiary amines differently. These differences are observed in the solubility of the sulfonamide product in alkali.

Comments