What is Ionic Bond?
An ionic bond is formed when an atom donates electrons and another atom accepts that electron. These ions are then held together by strong electrostatic forces of attraction. The overall charge of the compound is neutral although it consists of positively charged ions known as cations and negatively charged ions known as anions. On the other hand, a covalent compound is formed by mutual sharing of valence electrons in order to obtain stability. The shared electrons are known as electron pairs or bond pairs.
In ideal situations we can say that a bond is hundred per cent ionic or covalent in nature. But in reality, no bond is completely ionic or covalent. If we take for an instance, the covalent hydrogen molecule, some ionic characters will be observed. There is a partial existence of covalent characters in an ionic bond which is discussed by Fajans in the following rule:
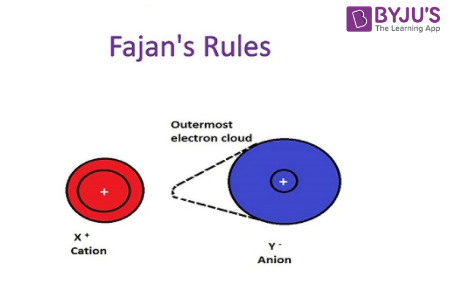
- The difference in the size of the ions will determine the percentage of covalent character in the ionic bond. For example, if the size of the cation is very small and that of the anion is very large. The ionic bond will show more covalent characters.
- The greater the charge present on the cation, more will be the covalent character of the ionic bond.
- The cations which have small size and charge like that of the transition metals with electronic configuration (n-1) dn ns0are more polarizing in nature as compared to the cations having noble gas electronic configuration ns2 np6(like alkali or alkaline earth metals).
The cations polarize the anions and try to pull the electronic charge towards themselves and this increases the electronic charge between the two ions. This is exactly what happens in a covalent bond, that is an electron charge density is build up around the nuclei. The percentage of covalent character in an ionic bond is determined by the polarization power of the cation, the polarizing ability of the anion and the distortion of anion taking place.
So far we have seen that no bond is perfectly ionic or covalent, there are covalent characters in an ionic bond and vice versa is also true. If you are curious to know more details on this topic, download BYJU’S – The Learning App.


Comments