What is Magnesium Chloride?
MgCl2 can be extracted from seawater or brine and is chemically named Magnesium Chloride.
- Magnesium Chloride is also called Magnesium dichloride or magnesium (II) chloride or Chloromagnesite and has one magnesium (Mg) and two chloride ions (Cl-).
- Magnesium dichloride and its salts are ionic halides and appear as thin white to gray granules.
- It is odourless and is highly soluble in water.
- It is widely used as a medicine for various cellular activities.
Table of Contents
- Properties of Magnesium Chloride- MgCl2
- Magnesium Chloride Structure- MgCl2
- MgCl2 Uses ( Magnesium Chloride)
- Magnesium Chloride Reactions
- Health hazards
- Problems faced in locomotive boilers
- Frequently Asked Questions
Properties of Magnesium Chloride – MgCl2
| MgCl2 | Magnesium Chloride |
| Molecular weight/molar mass of MgCl2 | 95.211 g/mol (anhydrous) |
| The density of Magnesium Chloride | 2.32 g/cm3 (anhydrous) |
| Boiling point of Magnesium Chloride | 1,412 °C |
| Melting point of Magnesium Chloride | 714 °C |
Magnesium Chloride Structure – MgCl2
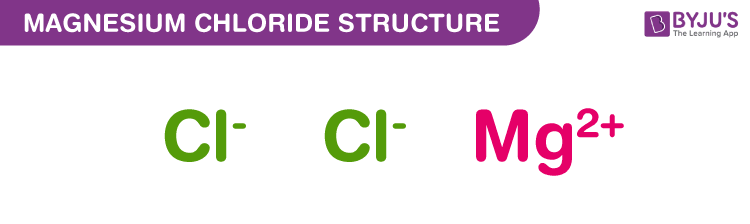
Magnesium Chloride Structure – MgCl2
MgCl2 Uses (Magnesium Chloride)
- Magnesium Chloride is used as a precursor for magnesium metals.
- Used for wind erosion, dust control, and soil stabilization.
- Used in fire extinguishers.
- Used as a food additive.
- Used in the manufacturing of paper.
- Used in making disinfectants.
- Used as a flocculating agent.
- Used as a catalyst.
- Used as thread lubricant.
Magnesium Chloride Reactions
-
- In the Dow process, magnesium hydroxide reacts with hydrochloric acid to regenerate magnesium chloride. The reaction is as follows:
Mg(OH)2(s) + 2 HCl → MgCl2(aq) + 2 H2O(l)
-
- MgCl2 as a precursor. The reaction is as follows:
Health hazards
Chloromagnesite is non-combustible but when heated liberates irritating toxic gas or fumes. Inhaling this compound causes severe coughing.
Problems faced in locomotive boilers
Well water consists of dissolved magnesium chloride. It was used in locomotive boilers located on the Trans-Australian Railway and resulted in serious and costly maintenance problems. This problem occurred during the steam era. There was no facility for freshwater watercourses, therefore they had to rely on bore water. The locomotive boilers could last for less than a quarter. The maintenance cost was expensive.
Frequently Asked Questions
What is magnesium chloride used for?
Magnesium is important to many body systems, particularly to the muscles and nerves. Magnesium chloride (lack of natural magnesium in the body) is used to treat or prevent magnesium deficiency. Magnesium chloride can also be used for non-listed purposes in this Drug Guide.
What is magnesium chloride made of?
Magnesium chloride Chloride. A compound inorganic consisting of one magnesium and two chloride ions. The compound is used as a source of magnesium ions in medicine which is important for many cellular activities. This was used as a cathartic and in alloys too.
What are the health benefits of magnesium chloride?
This drug is a mineral supplement used in the blood to prevent and treat small quantities of magnesium. Some products are also used to treat symptoms of excessive stomach acid such as stomach pain, heartburn and indigestion with acids.
Is magnesium soluble or insoluble?
The hydroxide of magnesium is insoluble in mud. However, the solution is slightly simple if it is shaken in water and filtered out. This means that in the solution there are more hydroxide ions than in the original water. This is due to a breakdown of some magnesium hydroxide.
Is magnesium chloride acidic or basic?
Solid magnesium chloride is an electrical nonconductor since the ions are not free to pass. But, as the ions are free on melting, it undergoes electrolysis. Magnesium chloride form neutral in the water. as it forms from strong acid HCl and strong base Mg(OH)2
Learn more about the Structure, physical and chemical properties of MgCl2 from the experts at BYJU’S.

Comments