What is the Maillard Reaction?
The Maillard reaction is an organic chemical reaction in which reducing sugars react with amino acids to form a complex mixture of compounds. This reaction is responsible for the characteristic flavour and aroma of browned food. The Maillard reaction is named after the French chemist Louis Camille Maillard. One of the first scientists who gave a more general idea of the reaction generating this type of food aromas was Louis Camille Maillard.
This reaction can be classified as a non-enzymatic browning reaction. The optimum temperature for this reaction ranges from 140oC to 165oC. It is important to note that caramelization is not a Maillard reaction (it proceeds at higher temperatures and involves the pyrolysis of sugars).
Several flavour compounds are formed during the Maillard reaction. These compounds can break down to yield other flavour compounds. Therefore, different types of food produce different flavour compounds while undergoing the Maillard reaction.
Mechanism of the Maillard Reaction
- The Maillard reaction mechanism begins with the formation of an N-substituted glycosamine (along with water) from the reaction between the amino group of the amino acid and the carbonyl group of the reducing sugar.
- Now, the glycosamine is transformed into ketosamines via Amadori rearrangement.
- These ketosamines undergo further reaction via several pathways and can form reductones, butanedione, methylglyoxal, and several other products of short-chain hydrolytic fission.
- The ketosamines can also go on to form melanoidins and other brown nitrogenous polymers. This imparts the characteristic brown colour to the food.
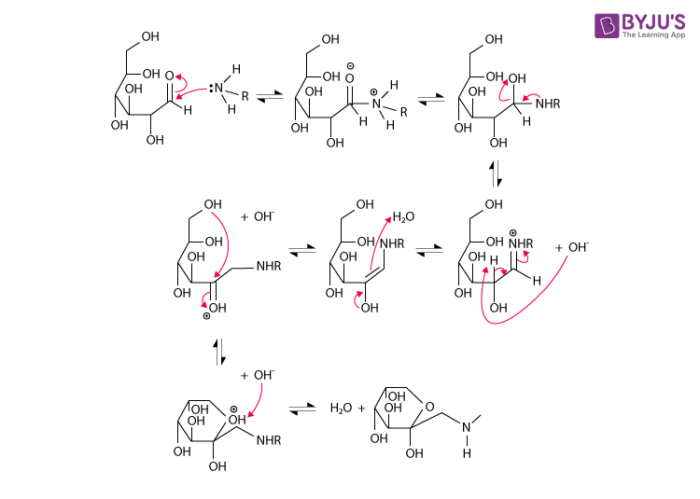
An illustration detailing the mechanism of the Maillard reaction is provided above. It is important to note that the Maillard reaction between amino acids and reducing sugars can result in the formation of Acrylamide (which is a possible carcinogen in humans). This compound is formed from the reaction between asparagine and dicarbonyl.
To learn more about the Maillard reaction and other important named reactions in organic chemistry, such as the Friedel-Crafts alkylation and acylation reactions, register with BYJU’S and download the mobile application on your smartphone.
Maillard reactions are non-enzymic browning reactions. In practice, any browning in foods is a Maillard reaction. The Maillard reaction between sugar and protein has been postulated as the cause for the browning and arrestment of carious lesions
Frequently Asked Questions – FAQs
What is the Maillard reaction and how does it occur?
The Maillard reaction is an organic named reaction which is named after the French chemist Louis Camille Maillard. It is sometimes referred to as non-enzymatic browning. The Maillard reaction is a relatively complex process that involves heat-induced chemical reactions between proteins and reducing sugars. The process begins with the formation of glycosylamine from the chemical reaction between an amine and reducing sugar.
List some examples of colour changes in food items due to the Maillard reaction.
Some common examples in which the Maillard reaction is responsible for the change in colours and flavours of food items include:
- The formation of caramel from sugar and milk.
- The browning of bread during the preparation of toast.
- The change in colour and flavour when meat is roasted.
- The change in colour during the preparation of condensed milk.
What are the key factors that affect the Maillard reaction?
Since the Maillard reaction involves the production of water, the reaction is inhibited by environments that have high water activity. It can also be noted that the amount of visible browning in the Maillard reaction varies based on the type of amino acid participating in the reaction. Finally, it is important to note that hexose sugars are more reactive toward the Maillard reaction than disaccharides and that pentose sugars are even more reactive than hexoses towards this named reaction.
How can the Maillard reaction be controlled?
The Maillard reaction can be controlled by changing the amount or the concentration of the reducing sugars present in the reaction environment. Alternately, this named reaction can be controlled by limiting the availability of the amino acids in the reaction. Examples of typical reducing sugars that participate in the Maillard reaction include fructose, glucose, maltose, lactose, and ribose.
What is the reason behind the browning of foods in Maillard reactions?
In Maillard reactions, the relatively reactive carbonyl group belonging to the reducing sugar undergoes a chemical reaction with the nucleophilic groups of the amino acid. This reaction triggers the change in colour and also facilitates the formation of many flavour compounds. However, it is important to note that energy must be supplied in the form of heat for this reaction to occur.
Why is the Maillard Reaction important?
The Maillard response is defined as the reaction between a diminishing sugar and an amino group and their subsequent interactions. It is also responsible for the development of carcinogens and might, by reducing the concentration of essential amino acids, also decrease the nutritious content of foods.
What happens during a Maillard chemical reaction?
The Maillard reaction is the chemical reaction that occurs in the presence of heat between amino acids and reducing sugars that results in food browning thereby producing fresh aromas and flavours. The building blocks of proteins which can be contained in our diet are amino acids.
How does temperature affect Maillard reaction?
In the cooking of nearly all forms of foods, the Maillard reaction happens, while the basic sugars and amino acids present create quite different aromas. The Maillard reaction is speeded up by high-temperature cooking because heat both increases the rate of chemical reactions and accelerates water evaporation.

Comments