What is Matter?
A matter is anything that has mass and occupies space. Pen, paper, clips, sand, air, ice, etc. are different forms of matter. Every matter is made up of tiny particles. These particles are so tiny that they can’t be seen with naked eyes. Let’s learn about the different characteristics of particles of matter.
Characteristics of Particles of Matter
As mentioned earlier, every substance is made up of particles. These particles exhibit some characteristics. They can influence the state and properties (physical and chemical) of a substance. The three characteristics shown by particles of matter are as follows.

Particles Have Space Between Them
There are small voids between every particle in a matter. This characteristic is the concept behind the solubility of a substance in other substances. Let’s try to understand this with an illustration.
Take a glass of water. Put a teaspoon of salt/sugar and mix them properly. You will observe that the water is still clear. This is because the particles of salt/sugar get into the interparticle spaces between the water particles. This proves that there are voids between particles of a substance. If you add more salt/sugar, it will dissolve until all the space between water particles gets filled.
Particles Are Constantly in Motion (or) Particles are Continuously Moving
Particles of the matter show continuous random movements. The kinetic energy they possess helps them in this movement. The spreading of ink in a beaker of glass, the smell that comes from agarbattis, etc. are few illustrations that show the movement of particles of a substance. When the particles of two different types of matter intermix on their own, the phenomenon is called diffusion.
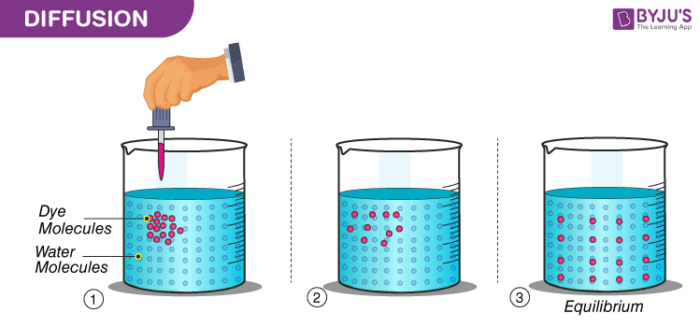
The diffusion of particles becomes fast when the temperature is increased. A rise in temperature increases the kinetic energy of the particles, making them move more vigorously.
Particles Attract Each Other
Take an iron rod, a stick of chalk, and a pen. Try to break each one of these. Which one of these is easy to break? The iron rod is stronger than the other two items. What makes an item stronger? Yes, it’s the particles in them which are held by the inter-particle force of attraction.
In every substance, there is an inter-particle force of attraction acting between its particles. To break something we need to overcome this force. The strength of the force differs from one substance to another.
The inter-particle force of attraction and the kinetic energy of the particles primarily determine the physical state of any matter.
Physical Properties Of Matter
Since a long time, scientists have been researching about chemical and physical properties of matter. In simple terms, they define matter as something which has mass and occupies space (that is volume). Hence, everything we see around us is matter. For example, stars that twinkle, the sun that shines, the food we eat, bricks we used to make buildings, water in oceans and rivers, rocks that form mountains, etc. All of these can be brought under a single umbrella called matter. But does all the matter that we see exhibit the same physical properties? Obviously not, a block of wood is continuous while sand particles are coarse. Similarly, salt particles are soluble in water while sand particles are not. All these properties are known as physical properties. Let us learn some physical properties through an activity.
- Let us take 5-6 crystals of potassium permanganate and dissolve them in 300 ml of water.
- Pour out 20 ml of this solution into 100 ml of pure water present in another beaker.
- Repeat the above procedure by again taking out 20 ml of the above solution and pouring it into 100 ml of pure water present in another beaker.
- Keep on repeating this procedure by diluting the solution like this 8 to 10 times.
- Observe the colour of the last solution.
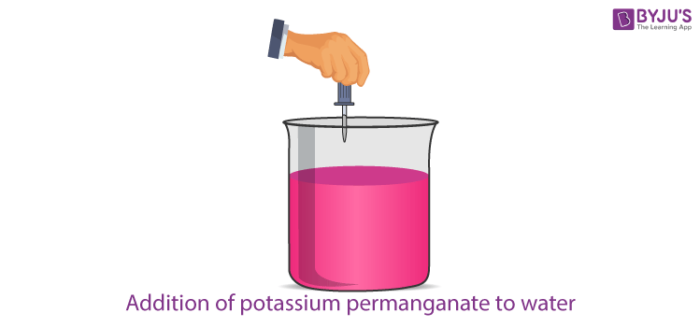
We observe that the solution in the last beaker is light pink. Thus, we can conclude that even a very small amount of potassium permanganate is able to change the colour of the solution. Thus, we can say that matter can be broken into millions of tiny pieces further. This piece is indestructible in nature.
Some physical properties of matter are
- All matter has mass and occupies some space.
- They can be broken into millions of tiny pieces further.
To learn more about atoms and molecules, and the inter-convertibility of matter from one form to the other, download BYJU’S – The Learning App.

so incredible on how your question is answered in a second with ease
It is very useful answer for me . Thank you Byjus to providing such a excellent answer . Thank you again. 😊