What is Methanol?
Methanol is simplest alcohol with chemical formula CH3OH. It is not a hydrocarbon since the hydroxyl group is chemically bonded to the carbon atom.
It consists of a methyl group linked with a hydroxyl group. It is also known as Wood alcohol or Methyl alcohol. It has a distinctive odour which is milder and sweeter than ethanol. It is volatile and does not have colour. It is a flammable, light, poisonous liquid. Consumption of methanol is toxic and can cause blindness. It is widely used in the manufacture of acetic acid and formaldehyde.
Robert Boyle was the first to isolate methanol in the year 1661. It was produced by the distillation of boxwood (Buxus). Nowadays methanol is prepared by the direct combination of carbon monoxide gas and hydrogen in the presence of a catalyst. It is a common laboratory solvent. It is a frequent denaturant additive in the manufacturing of ethanol.
Table of Contents
- Recommended Videos
- Properties of Methanol
- Methanol Structure
- Uses of Methanol
- Frequently Asked Questions – FAQs
Recommended Videos

Properties of Methanol – CH3OH
| CH3OH | Methanol |
| Molecular Weight/ Molar Mass | 32.04 g/mol |
| Density | 792 kg/m³ |
| Boiling Point | 64.7 °C |
| Melting Point | -97.6 °C |
Methanol Structure – CH3OH
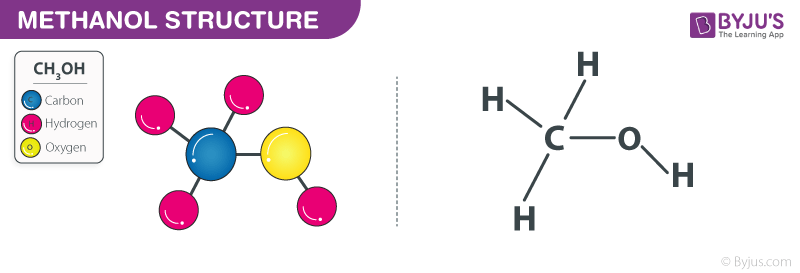
Methanol Structure – CH3OH
Uses of Methanol (CH3OH)
- It is used in polymers after getting converted to formaldehyde.
- It is used to produce hydrocarbons.
- It is used as a precursor for methyl ethers, methylamines, and methyl halides.
- It is used as a fuel for internal combustion engines.
- It is an excellent energy carrier.
- It is used in wastewater plants.
- It is used as fuel in boating stoves and camping.
- It is used as an antifreeze.
- It is used in the synthesis of chemicals.
- Pure methanol is used in the manufacture of perfumes, resins, and pharmaceuticals.
Frequently Asked Questions – FAQs
Why is methanol important?
In chemical synthesis pure methanol is an essential element. Methanol is also a high-octane, clean-burning fuel which in automobile vehicles is a potentially important substitute for gasoline. Wood-derived methanol is primarily used to make synthetic ethyl alcohol unsafe for drinking.
Why methanol is dangerous?
Methanol is extremely poisonous and flammable. Direct ingestion of more than 10mL can cause permanent blindness through optic nerve damage, central nervous system poisoning, coma and likely death. Such risks also occur when inhaling methanol vapours.
What does methanol smell like?
Methanol is flammable, and its vapours. It is a warm, volatile, flammable, colourless liquid with a distinctive smell similar to that of ethanol (drinking alcohol).
What product contains methanol?
It is an ingredient in consumer products such as antifreeze, glass cleaner, and paint thinners, although many people drink other, more harmless methanol-containing products daily. Methanol is present in fruit juice and spirits which are distilled naturally.
Is methanol an acid or base?
Methanol is both basic and acidic. Proton donors are acidic according to the Bronsted-Lowry method, and proton acceptors are bases. Methanol is a proton donor since it donates O-H proton to heavy bases like sodium hydride relatively quickly.
Register to BYJU’S to know more about the properties of CH3OH from the experts.

Comments