We all want to know that in a particular substance how many molecules are present. Molecules and atoms are very tiny, both in size and mass. The molar mass is the weight of one sample mole. Connect the atomic masses (atomic weights) of all atoms within the molecule to calculate the molar mass. Find the atomic mass for each element using the mass shown in the Periodic Table or Atomic Weight Table.
Multiply the subscript (number of atoms) times that element’s atomic mass and add the masses of all the elements in the molecule to obtain the molecular mass. Molar mass is typically expressed in either gram ( g) or kilograms (kg).
Table of Content
- What Is Molar Mass?
- Molar Mass Unit
- Mole Definition
- Recommended Videos
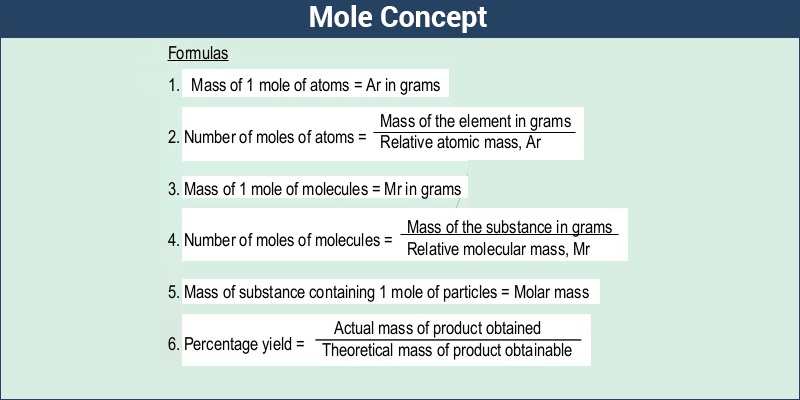
- Molar Mass Formula
- What is the Percentage of Composition?
- Solved Example
- Frequently Asked Questions – FAQs
What Is Molar Mass?
Molar mass of a substance is the mass in grams of one mole of the compound.
In a substance, the amount of entities present e.g. atoms, molecules, ions, is defined as a mole. A mole of any substance is 6.022×1023 molecules. Just as we take a standard value to calculate different things e.g. 1 dozen =12 items similarly we use the mole to calculate the size of the smallest entities quantitatively.
Molar Mass Unit
The standard unit for this is g mol−1. However, the SI unit is kg mol−1, which is very rare.
The number of atoms present in 12g (0.012 kg) of the 12C isotope is the number of particles present in 1 mole of the substance. One of the most important facts that should be kept in mind is that the mole of a substance always contains the same number of entities whatever the substance may be.
To know the number precisely the mass of the carbon-12 atom was calculated by using a mass spectrometer and it was found to be 1.992648×10-3g.
As we know that one mole of carbon weighs 12g, the total no. of atoms in it is equal to 6.0221367×1023. The no. of entities in 1 mole plays a vital role in calculations in chemistry. That is why it is given a name called Avogadro’s number(NA). From the above discussion, we can say that the number of atoms present in 1 mole of hydrogen is equal to 6.022×1023.
Mole Definition
In chemistry, the mole is a fundamental (SI) unit used to measure the amount of substance. This quantity is sometimes referred to as the chemical amount.
A substance is something that has mass and occupies space. The molar mass/molecular weight is actually the sum of the total mass in grams of the atoms present to make up a molecule per mole. The unit of molar mass is grams/mole.
Recommended Videos
Mole Concept, Atomic Mass, Molecular Mass & Molar Mass

Mass – Mole – Particles – Volume Conversions


Molar Mass – Formula
It is all very well to calculate the atomic molecular and formula masses of atoms, molecules, and other compounds, but since we cannot weigh an individual particle, these masses have limited usefulness. To make measurements of mass useful we must express chemical quantities at the macroscopic level. The bridge between the particulate and the macroscopic levels is molar mass, the mass in grams of one mole of a substance. The units of molar mass follow its definition; grams per mole. Mathematically, the defining equation of molar mass is
Molar mass = mass/mole = g/mol
The definition of atomic mass, the mole, and molar mass are all directly or indirectly related to carbon-12. This leads to two important facts.
- The mass of one atom of carbon-12 the atomic mass of carbon-12 is exactly 12 atomic mass units.
- The mass of one mole of carbon-12 atoms is exactly 12 grams; its molar mass is exactly 12 grams per mole.
Notice that the atomic mass and the molar mass of carbon-12 are numerically equal. They differ only in units; atomic mass is measured in atomic mass units, and molar mass is measured in grams per mole. The same relationship exists between atomic and molar masses of elements, between molecular masses and molar masses of molecular substances and between formula masses and molar masses of ionic compounds.
What is the Percentage of Composition?
We can find the percentage composition of a substance by dividing the mass of that substance by the total mass of the substance. Suppose we have to find out the percentage composition of hydrogen in butane(C4H10) then it will be:
The total mass of one mole of butane =58.123
Mass of hydrogen in one mole of butane = 10.0794
Therefore, the mass percent of hydrogen in butane
= 17.3 %
These concepts play a very important role in studying the behaviour of matter under different conditions.
Solved Example
Question:
What is the molar mass of sodium carbonate, Na2CO3?
Solution:
Since sodium carbonate contains two atoms of sodium, one atom of carbon and three atoms of oxygen. The molecular weight would be
Na : 2 x 23.0 = 46
C : 1 x 12.0 = 12
O : 3 x 16 = 48
When we add up the total values i.e, 46 + 12 + 48 = 106
Therefore, the molar mass of Na2CO3 is 106 g/mol.
Frequently Asked Questions – FAQs
Why do we need the Mole concept?
It allows the chemist to weigh quantities of two substances, say iron and sulphur, in order to obtain equal numbers of iron and sulphur atoms. A mole of a substance is known as a material mass containing the same number of basic units as atoms in exactly 12,000 g of 12C.
Why is Avogadro’s number called a mole?
The density of one mole in grams is the weight in atomic mass units of that element. The French physicist Jean Perrin called the number of units in the sum of one mole Avogadro a few years later. For example, one mole of water molecules contains 6.022140758 x 1023 molecules.
What is the use of the mole concept?
The mole is the chemical quantity unit. This connects the atom with the macroscopic quantities of material with which we work in the laboratory. It allows the chemist to weigh quantities of two substances, say iron and sulphur, in order to obtain equal numbers of iron and sulphur atoms.
What does Avogadro’s law state?
The law of Avogadro, also referred to as the rule of Avogadro or the theory of Avogadro, is an experimental gas law that relates the volume of a gas to the amount of gas present. The law of Avogadro states that “equal quantities of all gases have the same number of molecules at the same temperature and pressure.”
How many moles are in a mole?
The mole, abbreviated mol, is an SI unit that measures a specific substance’s number of particles. One mole is equal to 6.02214179 or other elementary units like molecules.
Know more about the mole concept and the related topics, for any further help contact the mentors at BYJU’S.

Comments