What is Molisch’s Test?
Molisch’s test is a chemical test which is used to check for the presence of carbohydrates in a given analyte. This test is named after Czech-Austrian botanist Hans Molisch, who is credited with its discovery. Molisch’s test involves the addition of Molisch’s reagent (a solution of α-naphthol in ethanol) to the analyte and the subsequent addition of a few drops of concentrated H2SO4 (sulphuric acid) to the mixture.
The formation of a purple or a purplish-red ring at the point of contact between the H2SO4 and the analyte + Molisch’s reagent mixture confirms the presence of carbohydrates in the analyte. An image detailing a positive result for Molisch’s test is provided below.
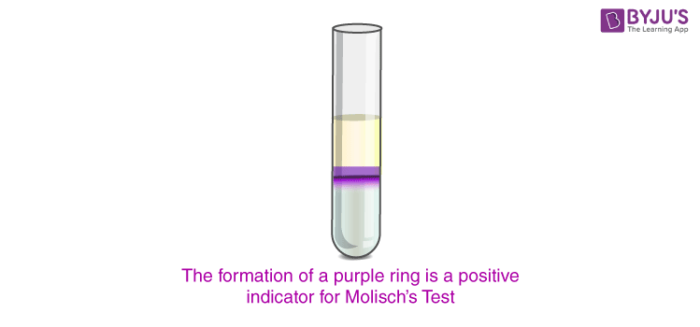
A positive reaction for Molisch’s test is given by almost all carbohydrates (exceptions include tetroses & trioses). It can be noted that even some glycoproteins and nucleic acids give positive results for this test (since they tend to undergo hydrolysis when exposed to strong mineral acids and form monosaccharides).
Table of Contents
Molisch’s Test Principle
In Molisch’s test, the carbohydrate (if present) undergoes dehydration upon the introduction of concentrated hydrochloric or sulphuric acid, resulting in the formation of an aldehyde. This aldehyde undergoes condensation along with two phenol-type molecules (such as α-naphthol, resorcinol, and thymol), resulting in the formation of a purple or reddish-purple coloured complex.
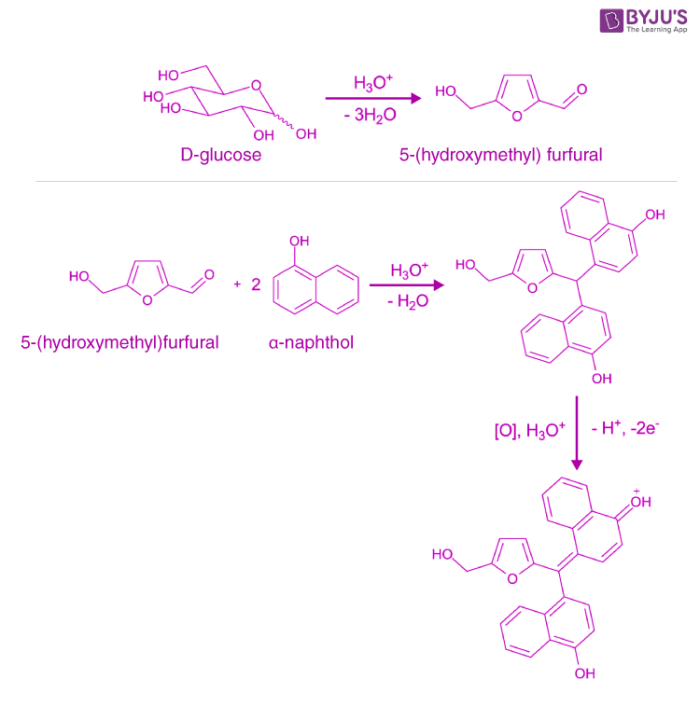
An illustration detailing the reactions undergone by D-glucose when it is subjected to Molisch’s test is provided above.
Molisch’s Test Procedure
2-3 drops of Molisch’s reagent must be added to a small amount of the analyte in a test tube and mixed well. Now, a few drops of concentrated sulphuric acid must be added drop-wise along the walls of the test tube to facilitate the formation of a layer and avoid mixing. The development of a purple ring at the layer formed by the concentrated acid is a positive indicator for Molisch’s test. If no purple or reddish-purple colour arises, the given analyte does not contain any carbohydrate.
Frequently Asked Questions – FAQs
What does Molisch’s test is used for?
Molisch’s test is a chemical test that detects the presence of carbohydrates in an analyte. This test is named after Czech-Austrian botanist Hans Molisch, who discovered it.
What is the principle of Molisch’s Test?
Molisch’s test is based on the dehydration of sulphuric acid into furfural. One hydroxyl group is removed from a sugar molecule when a sample containing carbohydrate molecules is treated with sulphuric acid and concentrated hydrochloric acid. Water is used to eliminate the hydroxyl group. After the hydroxyl group is removed from the sugar molecule, furfural is formed. The resulting furfural reacts with Molisch’s reagent (sulphonated – naphthol) to produce a purplish red product.
Which carbohydrate gave a positive result with Molisch’s test?
All carbohydrates (monosaccharides, disaccharides, and polysaccharides) pass the Molisch test. It is based on Sulphuric acid dehydrating the carbohydrate to produce an aldehyde, which condenses with two molecules of α-naphthol, resulting in the appearance of a purple ring at the interface.
What is the negative result of Molisch test?
The purple-coloured ring forms at the interface between the sulphuric acid and the test solution. Because the sulphuric acid is denser than the test solution, it remains above it. The lack of colour indicates a negative outcome.
What is the composition of Molisch’s reagent?
3.75 gm of α-naphthol in 25 ml of Ethanol 99% is the composition of Molisch’s reagent.
To learn more about Molisch’s test and other important chemical tests, such as the biuret test, register with BYJU’S and download the mobile application on your smartphone.

Comments