What is a Monomer?
Monomer is defined as a simple molecule with two or more binding sites through which it forms covalent linkages with other monomer molecules to form the macromolecule.
Monomers are thus building blocks of polymers. All simple molecules cannot behave as monomers but only those with two or more bonding sites can act as monomers. Thus molecules like ammonia, water, ethanol etc are not monomers. Alkenes, vinyl chloride, adipic acid, glycol with two bonding sites act as monomers.
Monomers and their dimer counterparts are archetypal plasmonic structures and a versatile theory could rightfully be expected to offer new insights for both individual monomers as well as for assemblies of such building blocks.
Table of Contents
Explanation of Monomers
Monomers and their dimer counterparts are archetypal plasmonic structures and a versatile theory could rightfully be expected to offer new insights for both individual monomers and for assemblies of such building blocks.
The following monomers are commonly used in the synthesis of acrylic solution polymers. Vinyl chloride and vinyl acetate monomers are excluded from this group of monomers as they are used in the manufacture of polyvinyl chloride and polyvinyl acetate polymers.
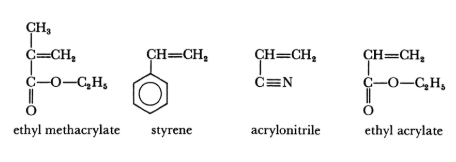
Acrylic solution polymers can be further subdivided into two distinct groups: thermosetting acrylics and thermoplastic acrylics. Thermosetting acrylics are polymers containing backbone monomers, which make up the bulk of the polymer together with at least one monomer which contains a reactive group, the latter will allow cross-linking through heat or with a catalyst.
The term includes a system that comprises a mixture of copolymer with a second compound or resin which will cross-link with it. Thermoplastic acrylics are prepared by the homopolymerisation or copolymerisation of a mixture of acrylic and methacrylic monomers and are usually considered relatively inert.
Classification of Monomer
Monomers are classified on the basis of their origin and synthesis are given below.
1. Classification Based on Origin
-
-
-
- Starches are polymers of monomer glucose.
- Cellulose is also a polymer of monomer glucose. It is made from the glucose produced during photosynthesis in plants.
- Protein is obtained as a result of polymerization of monomer o-amino acids.
- Synthetic polymers are man made polymers. For example, polythene, polystyrene, PVC, nylon and dacron.
-
-
2. Classification Based on Synthesis
Addition or chain polymers – It involves the repeated addition of monomers to the polymer chain. The monomers are unsaturated compounds.
The monomer and the chain growth polymerization compound is tabulated below.
| S.No | Monomers | Polymers |
| 1 | Ethylene | Polythene |
| 2 | Propylene | Polypropylene |
| 3 | Butadiene | Polybutadiene |
| 4 | Tetraflouroethylene | Polytetrafluoroethylene |
| 5 | Vinyl chloride | Polyvinyl chloride |
Natural Monomers
Natural monomers have been polymerized empirically for equally long periods for applications such as coatings, paint and ink setting, leather tanning, etc. Natural monomers with an unusual chemical structure for example, 4-hydroxyalkanoic acids, 5-hydroxyalkanoic acids and 6-hydroxyalkanoic acids that are synthesized by some microorganisms.
1. Amino acids
The name amino acid comes from the presence of an amino group and an acid carboxyl group (-COOH) in these molecules. Amino acids are the monomers which make up proteins. Amino acids are the monomer units. There are only 20 common amino acids found in these 10,000 proteins. True proteins contain only the elements carbon, hydrogen, oxygen, nitrogen, and sulphur.
2. Nucleotides
A chemical monomer unit of RNA (and informally of DNA). The monomer units of DNA are formally called deoxynucleotides. Polynucleotides are long polymers, made up of linear arrays of monomers called nucleotides, consisting of nitrogen bases (pyrimidines and purines) linked to sugar phosphate.
3. Glucose and Related Sugars
The repeating unit is glucose (C6H12O6), sugar monomers that are linked like beads on a string to form an almost endless chain. Protein polymers are similar, threadlike aggregates of as many as twenty types of amino acid monomers linked in series. And nucleic acids are much the same, long polymeric strands made up of a regularly alternating sequence of sugar and phosphate monomers with purine or pyrimidine base attached to each sugar and bending outward from the sugar phosphate backbone.
4. Isoprene
Isoprene is the monomer of natural rubber and naturally occurring terpenes and steroids, whereas 1,3-butadiene is a synthetic monomer used in the production of synthetic rubber. Isoprene is one of several related compounds, such as 1,3-butadiene and vinylcyclohexene, used in the rubber industry. Isoprene is also produced endogenously in rats and mice, an emission product of many plant species and the major endogenous hydrocarbon in human breath.
Frequently Asked Questions on Monomers
What are examples of monomers?
Examples of the monomers are glucose, vinyl chloride, amino acids, and ethylene. Every monomer can link up to form a variety of polymers in different ways. For example, in glucose, glycosidic bonds that bind sugar monomers to form polymers such as glycogen, starch, and cellulose.
What are the 4 types of monomers?
Monomers basically create blocks for molecules, including proteins, starch and many other polymers. Four big monomers are found: amino acids, nucleotides, monosaccharides, and fatty acids. The main forms of macromolecules are those monomers: proteins, nucleic acids, carbohydrates, and lipids.
What are monomers made of?
The term monomer originates from mono- (one) and -mer (part). Monomers are small molecules that can be joined to form more complex molecules called polymers in a repeated fashion. Monomers form polymers by the formation of chemical bonds or the supramolecular binding through a process called polymerization.
Is amino acid a monomer?
Amino acids do not have single monomers. They are basic compounds bound to the same molecule, with an amino group and a group of carboxylic acids. Instead, amino acids are monomers of proteins, long chains of amino acids that are bound together by amide bonds.
What are two monomers of carbohydrates?
Carbohydrates are among life’s four essential macromolecules. These are a polymer consisting of monomers known as monosaccharides. Easy sugars such as glucose and fructose are such building blocks. Two fused monosaccharides make a disaccharide.

Very useful app.