What is Sodium Bisulfate?
NaHSO4 is a sodium salt of the bisulfate anion with chemical name Sodium bisulfate. It is also called Sodium acid sulfate or Bisulfate of soda or Sodium hydrosulfate. In its anhydrous form, it is hygroscopic. The solutions are acidic with a pH value of 1 for every 1M solution.
Sodium acid sulfate is a dry granular compound white in colour. It dissolves in water and is corrosive to tissues and metals.
Table of Content
- Structure of Sodium bisulfate – NaHSO4
- Properties of Sodium Bisulfate – NaHSO4
- Production of Sodium hydrogen sulfate
- Chemical reactions of Sodium Bisulfate
- Uses of Sodium Bisulfate
- Health hazards associated with Sodium Hydrogen Sulfate
- Frequently Asked Questions-FAQs
Sodium bisulfate structure – NaHSO4
The structure of sodium bisulfate molecules (sodium hydrogen sulfate molecules) is illustrated below. It can be noted that this compound features an ionic bond between the positively charged sodium cation and the negatively charged bisulfate anion.
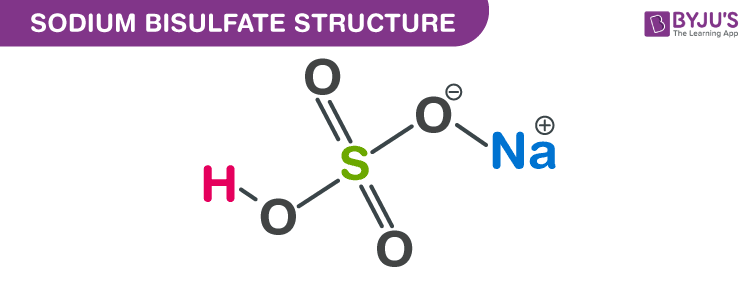
The exact mass and the monoisotopic mass of Sodium acid sulfate is 119.949 g/mol. The number of hydrogen bond acceptors equals to four and the number of hydrogen bond donor equals to one. This compound is canonicalized and has two covalently bonded units.
Properties of Sodium Bisulfate – NaHSO4
| NaHSO4 | Sodium bisulfate |
| Molecular weight of NaHSO4 | 120.06 g/mol (anhydrous) |
| Density of Sodium bisulfate | 2.742 g/cm3 (anhydrous) |
| Boiling point of Sodium bisulfate | Decomposes at 315 °C |
| Melting point of Sodium bisulfate | 150°C |
Production of Sodium Hydrogen Sulfate
At an industrial scale Bisulfate of soda is obtained as an intermediate via Mannheim process which involves the reaction of sodium chloride (NaCl) and sulfuric acid (H2SO4):
NaCl + H2SO4 → HCl + NaHSO4
The above reaction is an extremely exothermic reaction. The liquid NaHSO4 produced is sprayed and allowed to cool to form a solid bead.
Chemical reactions of Sodium Bisulfate
At 58 °C, the hydrated sodium bisulfate dehydrates and separates from the water molecule which is attached to it. It is allowed to cool. Heating it to 280 °C gives sodium pyrosulfate which is a colourless salt:
2 NaHSO4 → Na2S2O7 + H2O
Uses of Sodium Bisulfate-NaHSO4
- Sodium bisulfate is used to lower the pH of water in swimming pools.
- Used in metal finishing.
- Used in the chicken house to reduce the concentration of Salmonella.
- Used as a bleaching agent.
- Used as a catalyst.
- Used in the manufacturing of paper products.
- Used in the water treatment products.
- Used in paints.
- Used in agricultural chemicals.
- Used in velvet cloths.
Health hazards associated with Sodium Hydrogen Sulfate
Sodium hydrosulfate is a toxic, corrosive and non-combustible compound. Inhaling, swallowing or skin contact causes severe injury or leads to death. Oh heating, it liberates irritating and toxic gases.
Frequently Asked Questions-FAQs
1. What is the use of sodium bisulphate?
Sodium bisulfate is used as a bleaching agent, to lower the pH of water in swimming pools, water treatment products and manufacturing of paper products etc.
2.What is the sodium bisulfite test used for?
Sodium bisulfite test used to identify the presence of carbonyl group in the compound. A saturated solution of sodium bisulfite is taken in a clean test tube. 1ml of the given organic compound added to be tested. Shake well and leave it for 15-20 minutes. If there is a formation of white crystalline precipitate then the presence of the carbonyl group is confirmed.
3. What is the pH of sodium bisulfite?
40% aqueous solution of Sodium bisulfite is a pale-yellow liquid and the PH of the solution is 3.6 – 4.6.
4. Why is sodium bisulfite acidic?
Sodium bisulfite or sodium hydrogen sulfate is prepared by the reaction of sodium chloride (NaCl) and sulfuric acid (H2SO4) due to substitution of only one acidic proton of the diprotic sulfuric acid. It behaves as acid.
5. What are the properties of sodium bisulfite?
Sodium bisulfite is white solid, it is slightly sulfurous odour, soluble in water, the density of sodium bisulfite is 1.48 g/cm3. Melting point of sodium bisulfite is 150℃ and the boiling point is 315℃.

Comments