Nylon was discovered by Wallace Carothers in the year 1938, and its discovery led to the new age of fibres and textiles. Before its discovery of it, the thread that was used for clothing purposes was made of animal and plant fibre. But, the fibre obtained from plants and animals was weak in strength and could easily be rotted. The strongest fibre known at that time was silk. Carothers reasoned that a fibre having a strength similar to silk could be obtained when a polymer is bonded by an amide linkage. Nylon is very durable and has remarkable strength. As it is very strong, we can get very thin threads of nylon. The nylon fibre is used in making strong ropes and various fabrics.
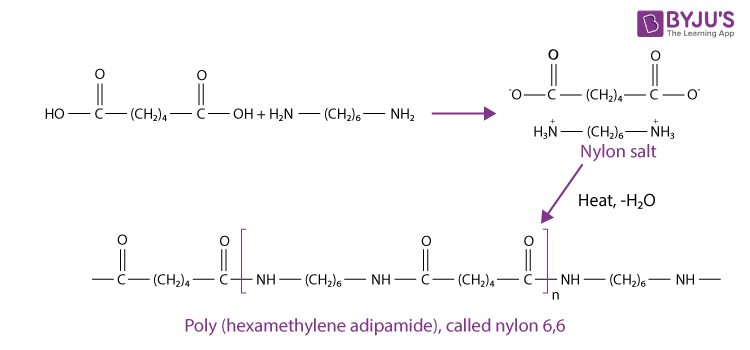
Polyamide is popularly known as nylon. Polyamides are made when a reaction takes place between diacids and amines. Nylon 6, 6 is the most common type of polyamide, and it is formed when six carbon diacid (adipic acid) reacts with six –carbon diamine. The six carbon diamine is commonly known as hexamethylene diamine. When adipic acid is mixed with hexamethylene diamine, this reaction gives a white solid, which is called nylon salt. We get molten nylon when the nylon salt is heated at 250°C, and molten nylon is cast into a solid shape. The following reaction takes place for the production of nylon 6,6.
Let us move on to the properties of nylon:
- It has a compact molecular structure.
- It provides high resistance against any plant, animal or fungi.
- It also provides resistance to abrasion.
- It has very high durability and can be elongated to the extent desired by the manufacturer.
These properties of it make it very user-friendly and hence it is very famous in the clothing sector. A fibre of it is converted into a cloth and many more articles. The clothes are very famous because of their lightweight and durability. Its main uses of it are as follows:
- In the automobile industry, resins of it are used in the engine compartments.
- The comb which is used daily by human beings is made up of solid nylon.
- Nylon resins are also used in food packing films to provide an oxygen barrier and save the food from getting oxidized or destroyed.
These are only a few major uses of nylon. It has been a boon for the textile industry and has had a great impact on human life. It is a synthetic polymer which is in great demand. After discussing so much on fibre, let us move on to polyester, which is also a very important material and is also highly used in today’s world.
Polyester: Polyesters are formed as a result of condensation of dicarboxylic acids and diols. The most popular example of polyester is terylene. When ethylene glycol and terephthalic acid are heated at 420 to 460 K in the presence of zinc acetate-antimony trioxide catalyst, the polyester terylene is manufactured. Polyester offers great durability and is resistant to most chemicals. It is resistant to stretching and shrinking and does not get wrinkled. Polyester is hydrophobic in nature. Hence it dries up quickly. These reasons have made polyester a very popular fabric and are largely used in the textile industry today. The usage of polyester can be classified as:
- Apparel: The different types of clothing are made from polyester, which is a very famous fabric in the textile industry.
- Home furnishing: The basic furniture, for example, carpets, curtains, bed sheets, pillow covers, wall hangings etc., are made up of polyester.
- Other applications: Polyester is used as a raw material in many articles, for example, hoses, floppy disk liners, ropes and nets, thread, auto upholstery etc.
So far we have seen the description of nylon, polyester and the various uses of polyester. For further details on these topics, kindly install BYJU’s learning app and enjoy the journey of learning in a new style.

Comments