What is Oxalate?
C2O4−2 is a dicarboxylic acid dianion with chemical name Oxalate.
Oxalate is also called Ethanedioate or Oxalate Ion or Oxalic Acid Dianion. It is obtained by deprotonation of both the carboxy groups of C2H2O4 (oxalic acid). It is widely used for derivatives, such as salts of oxalic acid, for example, dimethyl oxalate or sodium oxalate. It also forms coordination compounds and is sometimes abbreviated as ox.
Ethanedioate is found in many plants and is obtained by the incomplete oxidation of carbohydrates. The roots and leaves of buckwheat and rhubarb are some examples of oxalate-rich plants. It plays a role by being a plant metabolite or a human metabolite.
Properties of Oxalate – C2O4−2
| C2O4−2 | Oxalate |
| Molecular weight of C2O4−2 | 88.019 g/mol |
| No. of hydrogen bond acceptor | 4 |
| Monoisotopic mass of Oxalate | 87.98 g/mol |
| No. of hydrogen bond donor | 0 |
Oxalate Structure – C2O4−2
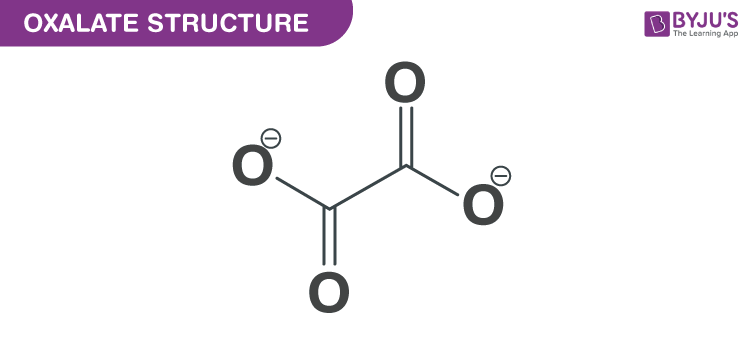
Oxalate Structure
Structure of Oxalate
With the help of X-ray crystallography, simple oxalate salts appear as a planar conformation with D2h molecular symmetry, and with a conformation where the O–C–C–O, dihedrals approach at a 90° angle along with an approximate D2d symmetry.
C2O4−2 Uses (Oxalate)
- Oxalate such as calcium oxalate is used in the manufacturing of ceramic glazes.
- Escitalopram Oxalate is used in the treatment of anxiety and depression.
- In its powdered form, it is used as a pesticide in beekeeping.
- It acts as an excellent ligand for metal ions.
Health Hazards
Oxalic acid in the human body combines with divalent metallic cations such as iron (II) and calcium and form crystals of the corresponding oxalates. These crystals are then excreted from urine as minute crystals. These oxalates may form larger kidney stones which can obstruct the kidney tubules. Approximately 80% of kidney stones are formed due to calcium oxalate.
Frequently Asked Questions – FAQs
What are oxalate ions?
It is also called Ethanedioate or Oxalate Ion, or Dianion of Oxalic Acid. This is obtained by deprotonation of both C2H2O4 (oxalic acid) carboxy groups. It is commonly used, for example, for compounds such as oxalic acid salts, dimethyl oxalate, or sodium oxalate.
What is the charge on oxalate ion?
C2O4 is a polyatomic ion with a -2 charge. Within this molecule, the oxygen atom has an oxidation state of-2 as oxygen also has -2 charges on it.
Is oxalate the same as oxalic acid?
For nutrition science, the words “oxalic acid” and “oxalate” are used interchangeably. Once ingested, oxalate can bind to minerals to form compounds, including oxalate in calcium and oxalate in iron. It occurs mainly in the colon but can also occur in the kidneys and other areas of the urinary tract.
What is oxalate used for?
Oxalic acid can also be produced by ethylene glycol (“antifreeze”), glyoxylic acid, or ascorbic acid (vitamin C) metabolism. Powdered oxalate is used in beekeeping as a pesticide for combating the bee mite.
What is C2O4 called?
C2O4−2 is a chemical-named dianion of a dicarboxylic acid, Oxalate. It is also called Ethanedioate or Oxalate Ion, or Dianion of Oxalic Acid.
Learn more about the Structure, physical and chemical properties of C2O4−2 from the experts at BYJU’S.

Comments