Table of Contents
- What is Phthalimide?
- Preparation of Phthalimide
- Reactions of Phthalic Anhydride
- Phthalimide Uses
- Limitation of the Reaction
- Frequently Asked Questions on Phthalimide
What is Phthalimide?
Phthalimide is acidic, since its conjugate base is resonance stabilised. Bromine is an electrophile, since it is electronegative and bromide is a good leaving group. Sodium hydroxide is a good base. The Phthalimide group is the most acidic site and hydroxide is the strongest base. Bromine is the only electrophile.
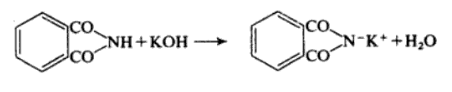
Phthalimide is readily deprotonated by hydroxide to give the corresponding anion, this reacts with bromine to give the N-bromophthalimide product. Gabriel Phthalimide Synthesis is a method of obtaining primary aliphatic amines . In this reaction phthalimide is converted into its potassium salt by treatment with alcoholic KOH .
Preparation of Phthalimide
Phthalimide may be prepared by heating phthalic anhydride with aqueous ammonia.
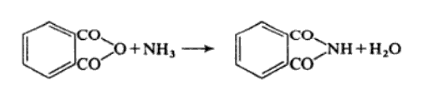
Alternatively Phthalimide may be prepared by fusing the anhydride with ammonium carbonate. It is weakly acidic with ethanolic potassium hydroxide it forms potassium Phthalimide.
The anhydride of phthalic acid is a key intermediate in the chemical industry and is obtained by oxidation of naphthalene in the chemical industry and is obtained by oxidation of naphthalene with fuming sulphuric acid and mercury catalyst or by the vapour phase oxidation of naphthalene with atmospheric oxygen.
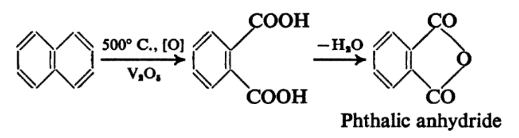
While phthalic anhydride is readily formed from phthalic acid the isomeric carboxylic acids do not give any anhydrides.
Reactions of Phthalic Anhydride
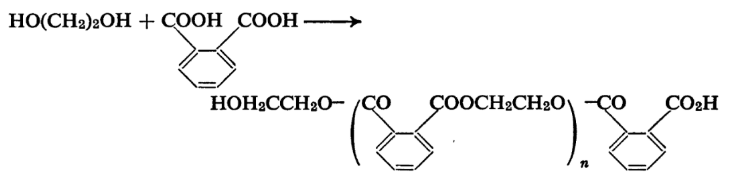
Esters of phthalic acid are obtained by the reaction of phthalic anhydride or the acid with alcohol and are used in diffusion pumps and in place of mercury in manometers. The diethyl and dihexyl esters are very widely used for this purpose. Dimethyl Phthalate is useful as an insect repellent. Phthalic anhydride is extensively used in the manufacture of the so-called alkyd resins. These resins are polyesters of acids containing two carboxyl groups and polyhydric alcohols. The polymer produced by the action of phthalic acid on ethylene glycol may be given as below.
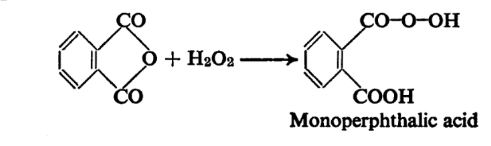
The imino hydrogen atom between the two carbonyl groups is distinctly acidic and hence phthalimide forms metallic salts. Gabriel’s synthesis of amines and amino acids, it may be recalled, employs the potassium salt of phthalimide. The Hofmann degradation of phthalimide as mentioned provides a convenient method of preparation of anthranilic acid. When phthalic anhydride is treated with alkaline hydrogen peroxide in the cold and the mixture then acidified monoperphthalic acid is obtained.
Phthalimide Uses
- Phthalimide analogues have been extensively used in medicinal chemistry owing to their wide range of applications as anticonvulsant, antiinflammatory, analgesic, hypolipidemic and immunomodulatory activities.
- Number of anti-inflammatory phthalimide analogues have been synthesised as tumor necrosis factor alpha inhibitors.
- It promotes the inflammatory response leading to many of the clinical problems associated with autoimmune disorders like rheumatoid arthritis, Crohn’s diseases, ankylosing spondylitis, psoriasis and refractory asthma.
- It is used in the synthesis of indigo and for preparing thiosalicylic acid, which is used in the synthesis of certain indigoid vat dyes.
Limitation of the Reaction
- The composition of the mixture obtained varies with the ratio of the reactant. However, the primary amine predominates if the large excess of ammonia is used. Because it is difficult to separate the mixture the method is not of much practical importance. This reaction is a typical example of a nucleophilic substitution reaction.
- The method is not suitable for the preparation of aniline and other aromatic amines because the corresponding haloarenes are very little reactive compared with alkyl halides. The cleavage of C-X bond does not take place by the nucleophilic attack of NH3.
Frequently Asked Questions on Phthalimide
What is the use of Phthalimide?
Phthalimide is used as a precursor to anthranilic acid, an azo dyes precursor and saccharin. Alkyl phthalimides are useful precursors to amines in chemical synthesis, particularly in peptide synthesis where they are used to “block both hydrogens and prevent substrates from being racemised.”
What is an amide group?
An amide is a functional group comprising a group of carbonyls connected to a nitrogen atom or some compound containing the functional group of amide. Amides are derived from an amine and a carboxylic acid. Amide is the name for the NH2 inorganic anion, too.
How do you make phthalic anhydride?
Catalytic oxidation of ortho – xylene or naphthalene is usually obtained by phthalic anhydride. A series of “switch condensers” are needed to isolate the phthalic anhydride from production by products such as o – xylene in water, or maleic anhydride. Phthalic anhydride can be made from phthalic acid, too.
Why is Phthalimide acidic?
Phthalimide is strongly acidic in nature since the proton is quickly donated and water soluble salts are formed with stronger bases.
How is Phthalimide formed?
Phthalimide can be prepared by heating alcoholic ammonia with phthalic anhydride, which yields 95–97 per cent. Alternatively, it can be prepared by taking ammonium carbonate or urea to treat the anhydride. It may also be caused by o-xylene ammoxidation.

Comments