Alcohols are compounds which contain one or more hydroxyl (-OH) groups directly attached to a carbon chain. Alcohols in the free-form are not a common occurrence in nature; they are found mainly in the essential or volatile oils obtained from flowers, leaves, and stems of the plants.
Table of Contents
General Methods of Preparation of Alcohols
1. Hydrolysis of Halides
Alkyl halides when boiled with an aqueous solution of an alkali hydroxide give alcohol through nucleophilic substitution mechanism.
R-X + KOH → R-OH + KX
This general procedure produces primary and secondary alcohols. Glycerol can be synthesized from propylene by a series of reactions including the hydrolysis of a halide as one step in the process.
2. Hydration of Alkenes
Direct hydration takes place by adding water in the presence of a catalyst.
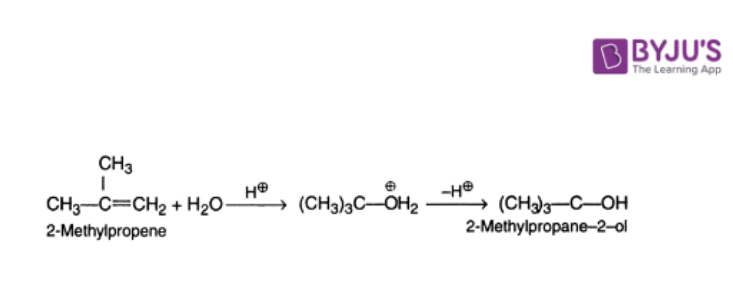
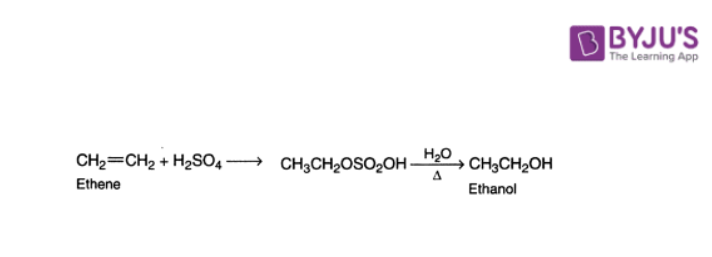
Indirect hydration is achieved by the addition of sulphuric acid to alkane followed by hydrolysis of the alkyl hydrogen sulfate.
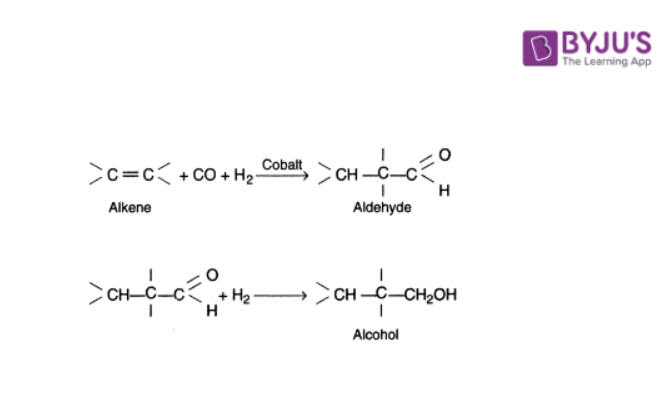
3. Hydroformylation of Alkenes
Lower molecular weight olefins react with carbon monoxide and hydrogen in the presence of a catalyst in a reaction called hydroformylation or the oxo reaction.
The resulting aldehyde is subsequently hydrogenated to form an alcohol.
4. Hydroboration of Alkenes
Alkenes, when treated with diborane, give alkyl boranes, R3B. Alkylboranes on oxidation with alkaline hydrogen peroxide give alcohol.
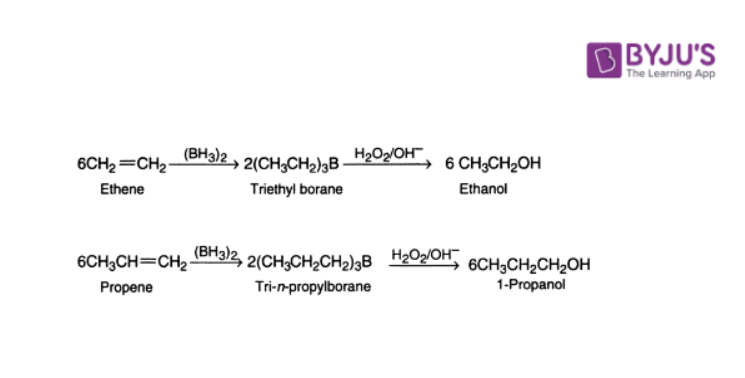
It is significant to note that this method always leads to anti-Markovnikov’s addition of water to alkenes.
5. Grignard Synthesis
All three types of alcohol (primary, secondary and tertiary) can be prepared from the Grignard reagents by interaction with suitable carbonyl compounds.
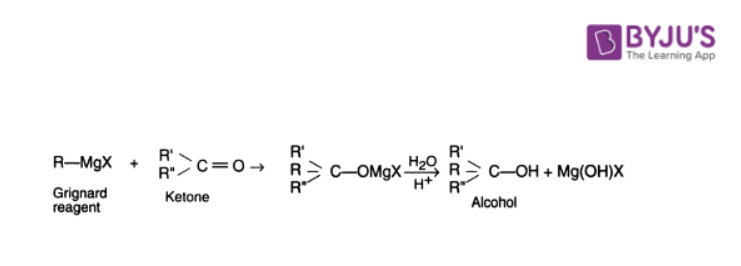
The reaction of the Grignard reagent with formaldehyde leads to primary alcohols, that with aldehydes other than formaldehyde yield secondary alcohols and that with ketones give tertiary alcohols.


Comments