Introduction
This topic explains the Resonance effect or Mesomeric effect and its types. In organic chemistry, the behaviour of electrons differs when elements other than that of the carbon atoms and hydrogen actively take part in the formation of molecular bonds.
The electronic factors influencing the organic reactions include the electromeric effect, the inductive effect, resonance effects, hyperconjugation, etc. All these factors relate to the organic molecules, in a diverse manner. Most of the biological molecules consist of a combination of these six elements: carbon, nitrogen, hydrogen, oxygen, sulphur, and phosphorus. Yet, they do not prevent the organic compounds from taking on the diverse properties of their chemical reactivity and physical characteristics.
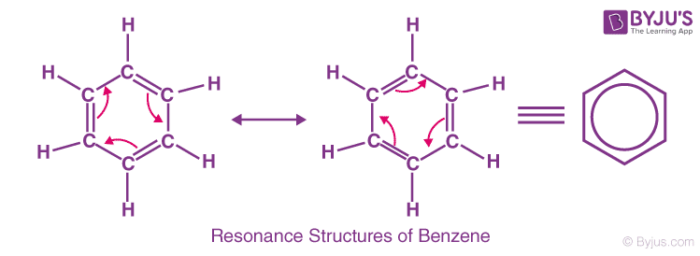
Organic molecules have a feature known as resonance or mesomerism. The delocalized electrons within some compounds where one single Lewis structure does not express the bonds are described by the factor termed resonance or Mesomerism in organic chemistry. The delocalized electrons in an ion or molecule can be represented by providing multiple structures known as resonances.
Table of Contents
Recommended Videos
Resonance Effect or Mesomeric effect In Chemistry
Definition
- Resonance Effect – The polarity induced in a molecule by the interaction of a lone pair of electrons with a pi bond or the interaction of two pi bonds in nearby atoms is known as the resonance effect.
- Mesomeric effect – The mesomeric effect is the polarity that develops in a molecule as a result of interaction between two -bonds or a -bond and a lone pair of electrons.
The main difference between resonance and the mesomeric effect is that the resonance effect describes how a molecule’s lone pair of electrons and bond pair of electrons determine its chemical structure, whereas the mesomeric effect describes how a molecule’s chemical structure is stabilized by using a functional group.

Resonance structures of several functional groups
The diagram above depicts the various resonance configurations of several substances with resonance effects.
The main difference between resonance types is the electron configuration. Because resonance structures clearly exhibit bonding in molecules, they provide a better representation of a Lewis dot structure. The more resonance structures a molecule possesses, the more stable it becomes. Mesomeric effects are caused by π-electron delocalization and contribute significantly to changes in acid and base strength caused by remote substituents, particularly via double bonds in conjugation with the ionisable centre, such as ortho or para (but not meta) substituents in aromatic or heteroaromatic compounds.
Resonance Effect Types
Positive resonance effect and negative resonance effect are the two types of resonance effects.
-
-
- Positive Resonance Effect- The electrons are transferred away from an atom or substituent group bound to the conjugated system in this process. For example – -OH, -SH, -OR, -SR.
- Negative Resonance Effect- The electrons are transferred towards the atom or substituent group associated with the conjugated system in this effect. For example – -NO2, C=O, -COOH, -C≡N.
-

Your website is very good. Your answer is very unique and easy .