What is Robinson Annulation?
Robinso,n Annulation is a useful organic reaction. This reaction is named after a British chemist Sir Robert Robinson. The term ‘annulation’ stands for ‘building a ring’. In this reaction, the formation of α, β-unsaturated cyclic ketones from methyl vinyl ketones and aldehyde or ketones takes place.
Actually, this reaction is a combination of two reactions. One is ‘Michael Addition’ and the other is ‘Aldol Condensation’.
For example, consider the following reaction.
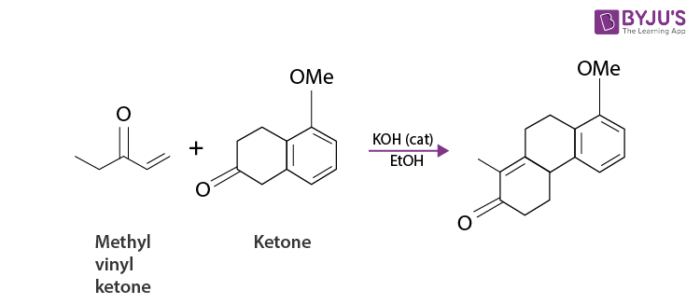
Robinson Annulation which normally involves a more highly substituted enolate would not be useful. Annulation of ketone with a fully substituted beta carbon by the Robinson method militates against steric hindrance. On the other hand, such a ketone undergoes alpha-methylenation without danger of isomerization and the annulation based on the 3C+3C condensation is viable.
Table of Contents
-
Robinson Annulation Mechanism
The synthetic outcome of a Micheal addition and subsequent intramolecular aldol condensation is called a Robinson Annulation.
- The Robinson annulation begins with a Micheal addition followed by aldol condensation.
- In the first step, Micheal addition takes place to an α,β – unsaturated ketone.
- Enolate is formed and tautomerization takes place for further reaction.
- Cyclization takes place. That is, aldol condensation reaction to form the six-membered cyclic product.
- Further hydrolysis results in the formation of α,β – unsaturated cyclic ketones.
Applications of Robinson Annulation
1. Robinson annulation is also used for synthesizing spirocyclic compounds.
2. The merits of the Robinson Annulation lie in its applicability in the total synthesis of complex molecules, and it continues to be the strategy of choice for the synthesis of naturally occurring steroids, alkaloids and terpenoids.
3. Since Robinson Annulation is considered a formal [4+2] condensation reaction the new rings formed by the reaction are always six-membered rings.
4. Robinson annulations have been broadened to encompass [3+3] annulations. In general, all ring-forming cascades involving the sequence of Micheal and intramolecular aldol reaction are classified as Robinson annulation.
5. Innumerable syntheses are hinged on the Robinson annulation. Noteworthy are those employing the Robinson annulation to construct an internal ring. The presence of a functionalized chain at the alpha carbon of the vinyl ketone generally simplifies a synthetic route. Robinson Annulation is an extraordinarily important synthesis of the six-membered ring.

