
Introduction
Rutherford was always curious in knowing about the arrangement of electrons in an atom. By performing an experiment using alpha particles and gold foil he came to some conclusions.
Experiments performed
Let us first learn something about the experiments he performed:
- A 1000 atoms thick gold foil was selected because he wanted as thin a layer as possible.
- Alpha particles are nothing but doubly charged helium ions. As its mass is 4u, the fast-moving alpha particles have a good amount of energy.
- He also expected that the alpha particles will be deflected as they are heavier than the protons. But what he observed was completely unexpected, he made the following observations:
-
- Most of the alpha particles passed straight through that gold foil.
- There was a deflection at a small angle by some of the alpha particles.
- A very small amount of alpha particles rebounded.
Rutherford concluded the following points after the observation:
- As there was very less deflection of alpha particles so he concluded that most of the space was empty in an atom.
- He also concluded that positive charge occupies a very less amount of space in an atom as very few particles were deflected from their path.
- A very small amount of alpha particles deflected with an angle of 1800, which indicated that the mass of the atom and the positive charge was concentrated at a small volume in an atom.
From all these observations he calculated that the radius of the nucleus is around 105 times less than the radius of the atom.
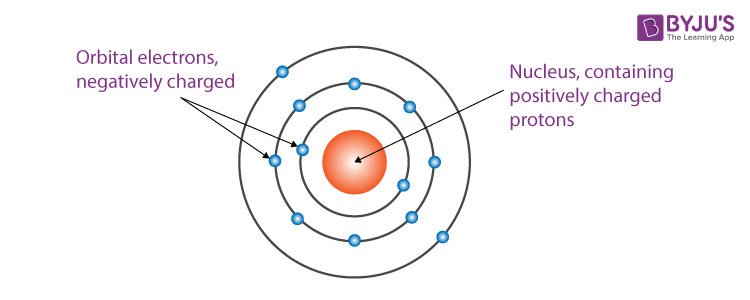
The following final model was put by Rutherford after all the observations:
- The nucleus is at the centre and is positively charged and nearly all the mass of the nucleus resides in the nucleus.
- Around the nucleus, electrons revolve in a circular path.
- The size of the nucleus is very less as compared to the size of the atom.
To learn more about Rutherford’s atomic model, register with BYJU’S now!

Comments