SN1 reaction mechanism follows a step-by-step process wherein first, the carbocation is formed from the removal of the leaving group. Then the carbocation is attacked by the nucleophile. Finally, the deprotonation of the protonated nucleophile takes place to give the required product. The rate-determining step of this reaction depends purely on the electrophilicity of the leaving group and is not impacted at all by the nucleophile.
Table of Contents
- What is an SN1 Reaction?
- SN1 Reaction Mechanism
- Stereochemistry of SN1 Reaction
- Recommended Videos
- Frequently Asked Questions – FAQs
What is an SN1 Reaction?
The SN1 reaction is a nucleophilic substitution reaction where the rate-determining step is unimolecular. It is a type of organic substitution reaction. SN1 stands for substitution nucleophilic unimolecular. Thus, the rate equation (which states that the SN1 reaction is dependent on the electrophile but not on the nucleophile) holds in situations where the amount of the nucleophile is far greater than the amount of the carbocation intermediate.
This reaction involves the formation of a carbocation intermediate. It is generally seen in the reactions of tertiary or secondary alkyl halides with secondary or tertiary alcohols under strongly acidic or strongly basic conditions. The SN1 reaction is often referred to as the dissociative mechanism in inorganic chemistry. Given below are some examples of an SN1 type of nucleophilic substitution reaction.
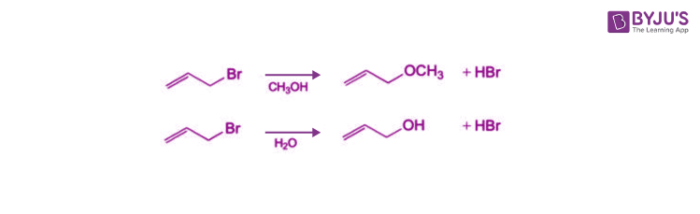
Effect of Solvent
- A solvent that can facilitate the formation of the carbocation intermediate will speed up the rate-determining step of the SN1 reaction.
- The preferred solvents for this type of reaction are both polar and protic.
- The polar nature of the solvent helps to stabilize ionic intermediates whereas the protic nature of the solvent helps solvate the leaving group.
- Examples of solvents used in SN1 reactions include water and alcohol. These solvents also act as nucleophiles.
SN1 Reaction Mechanism
Taking the hydrolysis of tertiary butyl bromide as an example, the mechanism of the SN1 reaction can be understood via the following steps.
Step 1
- The carbon-bromine bond is a polar covalent bond. The cleavage of this bond allows the removal of the leaving group (bromide ion).
- When the bromide ion leaves the tertiary butyl bromide, a carbocation intermediate is formed.
- As mentioned earlier, this is the rate-determining step of the SN1 mechanism.
- It is important to note that the breaking of the carbon-bromine bond is endothermic.
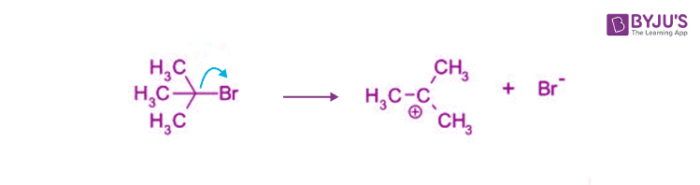
Step 2
- In the second step of the SN1 reaction mechanism, the carbocation is attacked by the nucleophile.
- Since water is used as a solvent, an oxonium ion intermediate is formed.
- Since the solvent is of a neutral nature, a third step where deprotonation occurs is necessary.

Step 3
- The positive charge on the carbocation was shifted to the oxygen in the previous step.
- The water solvent now acts as a base and deprotonates the oxonium ion to yield the required alcohol along with a hydronium ion as the product.
- Step 2 and Step 3 of this reaction are fast.

Stereochemistry of SN1 Reaction
The carbocation intermediate formed in step 1 of the SN1 reaction mechanism is an sp2 hybridized carbon. Its molecular geometry is trigonal planar, therefore allowing for two different points of nucleophilic attack, left and right.
If the reaction takes place at a stereocenter and if neither avenue for the nucleophilic attack is preferred, the carbocation is then attacked equally from both sides, yielding an equal ratio of left and right-handed enantiomers as shown below.
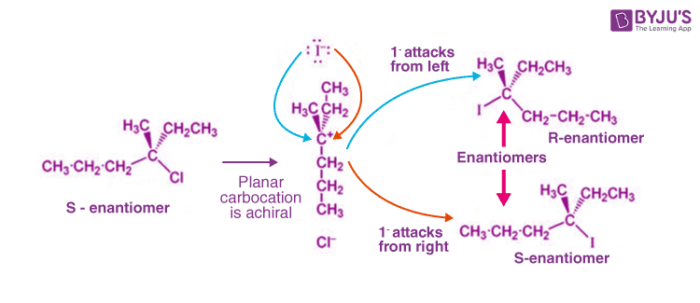
Thus, the tertiary/secondary alkyl halides can react with tertiary/secondary alcohols to undergo a nucleophilic substitution reaction. The halide is replaced with the nucleophile in the product.
Recommended Videos
SN1 & SN2 Mechanism

SN1 reactions – Reaction Mechansim

SN1 vs SN2 reactions

Frequently Asked Questions – FAQs
What is an SN1 reaction?
SN1 stands for substitution nucleophilic unimolecular. The SN1 reaction is a nucleophilic substitution reaction where the rate-determining step is unimolecular. It is a type of organic substitution reaction.
How many steps are there in the SN1 reaction?
SN1 reaction takes place in two steps. In the first step leaving group leaves and the substrate forms a carbocation intermediate. While in the second step, the nucleophile attacks the carbocation intermediate forming the product.
What do SN1 reactions depend on?
SN1 reactions depend on one reactant’s concentration and are independent of the nucleophile’s strength.
What solvent is used in the SN1 reaction?
A polar protic solvent is used in the SN1 reaction as it stabilises the carbocation intermediate.
Why does SN1 favour weak nucleophiles?
A nucleophile is not involved in the rate-determining step. Thus, it is independent of the strength of the nucleophile.

Comments