What is Sodium cyanide?
NaCN is a cyanide salt, a sodium salt, and a one-carbon compound with the chemical name Sodium cyanide. It is also called Cyanogran, a Cyanide of sodium or Cyanobrik. It is extremely poisonous and inhibits various metabolic processes. It is widely used as a test reagent for the proper functioning of chemoreceptors and in industrial processes.
Cyanogran is a crystalline solid white in colour which can also be obtained in powder or lump solid form. It contains an equal number of cyanide anions and sodium cations.
Table of Contents
- Properties of Sodium cyanide – NaCN
- Sodium cyanide structure – NaCN
- NaCN Uses (Sodium cyanide)
- Production of Sodium cyanide
- Health hazards
- Frequently Asked Questions
Properties of Sodium Cyanide – NaCN
| NaCN | Sodium cyanide |
| Molecular weight/molar mass of NaCN | 46.006 g/mol |
| Density of Sodium cyanide | 1.6 g/cm³ |
| Boiling point of Sodium cyanide | 1,496 °C |
| Melting point of Sodium cyanide | 563.7 °C |
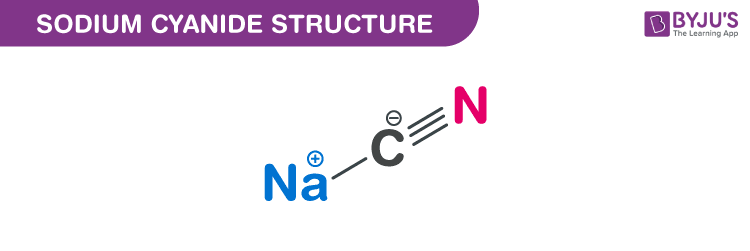
Sodium cyanide structure – NaCN
NaCN Uses (Sodium cyanide)
- Sodium cyanide is used in the extraction of gold.
- Used in the mining industry.
- Used as a rodenticide.
- Used to clean metals.
- Used in the manufacturing of dyes.
- Used in the making of electroplating solutions.
- Used in the production of hydrocyanic acid.
- Used in agricultural chemicals.
- Used to prepare nitriles.
- Used as a chelating agent.
Production of Sodium cyanide
Cyanide of sodium is obtained by reacting hydrogen cyanide (HCN) with sodium hydroxide (NaOH):
HCN + NaOH → NaCN + H2O
In the year 2006, worldwide production of Cyanobrik was estimated to approximately 5,00,000 tons. It was produced by the Castner process which involved the reaction of sodium amide (NaNH2) with carbon at high temperature.
Health hazards
Cyanogran is extremely toxic. The safe amount of oral lethal dose in humans is considered to be less than 5 mg/kg for a 70 kg person. This compound is fatal when inhaled, absorbed through the skin, or swallowed. Contact with it can cause burns to the eyes and skin. It is toxic when inhaled, ingested or in contact with vapours, or dusts and may result in death. On reacting with moist air or water it releases corrosive, flammable, toxic gases.
Frequently Asked Questions – FAQs
What are the uses of sodium cyanide?
Is sodium cyanide toxic?
How is sodium cyanide prepared?
Learn more about the Structure, physical and chemical properties of NaCN from the experts at BYJU’S.

Comments